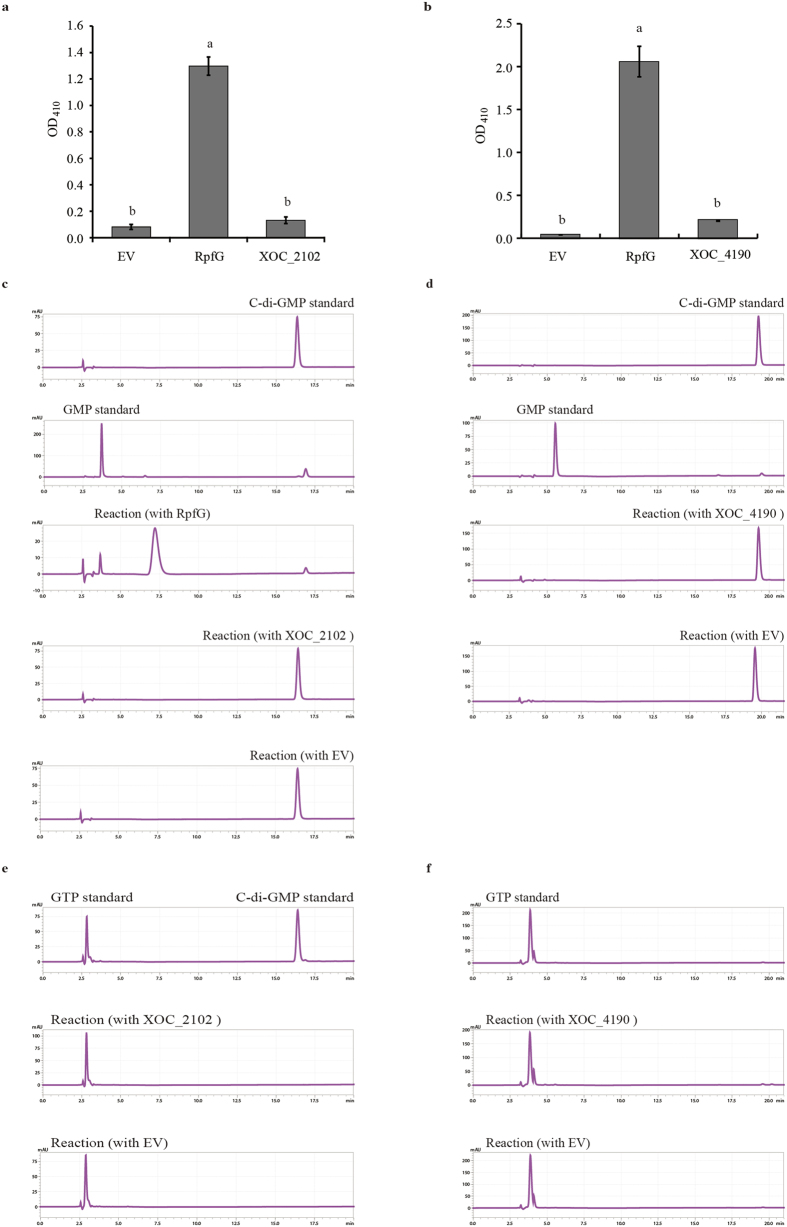
Figure 5. XOC_2102 and XOC_4190 are phosphodiesterase- and diguanylate cyclase-inactive.
The PDE activity of the purified XOC_2102 (a) and XOC_4190 (b) was detected by colorimetric assays. No yellow degradation product p-nitrophenol was detected when the purified XOC_2102 (a) or XOC_4190 (b) was incubated with the colorless phosphodiesterase substrate bis(p-nitrophenyl) phosphate. As a positive control, the known PDE RpfG degraded the substrate into the yellow product that was detected spectrophotometrically at 410 nm. The PDE activity of the purified XOC_2102 (c) and XOC_4190 (d) was detected by HPLC assays. No degraded product was detected when XOC_2102 and XOC_4190 were incubated with c-di-GMP. In the same reaction buffer, two hydrolytic products pGpG and GMP were detected after RpfG was incubated with c-di-GMP for 6 h (c). The DGC activity of XOC_2102 (e) and XOC_4190 (f) was assayed by HPLC. No synthetic c-di-GMP was detected when the purified XOC_2102 (e) and XOC_4190 (f) were incubated with GTP in the assay buffer for 6 h. GTP and c-di-GMP were loaded and detected as standards. The eluant from empty vector (EV)-transformed E. coli cells through nickel column was used as a negative control.