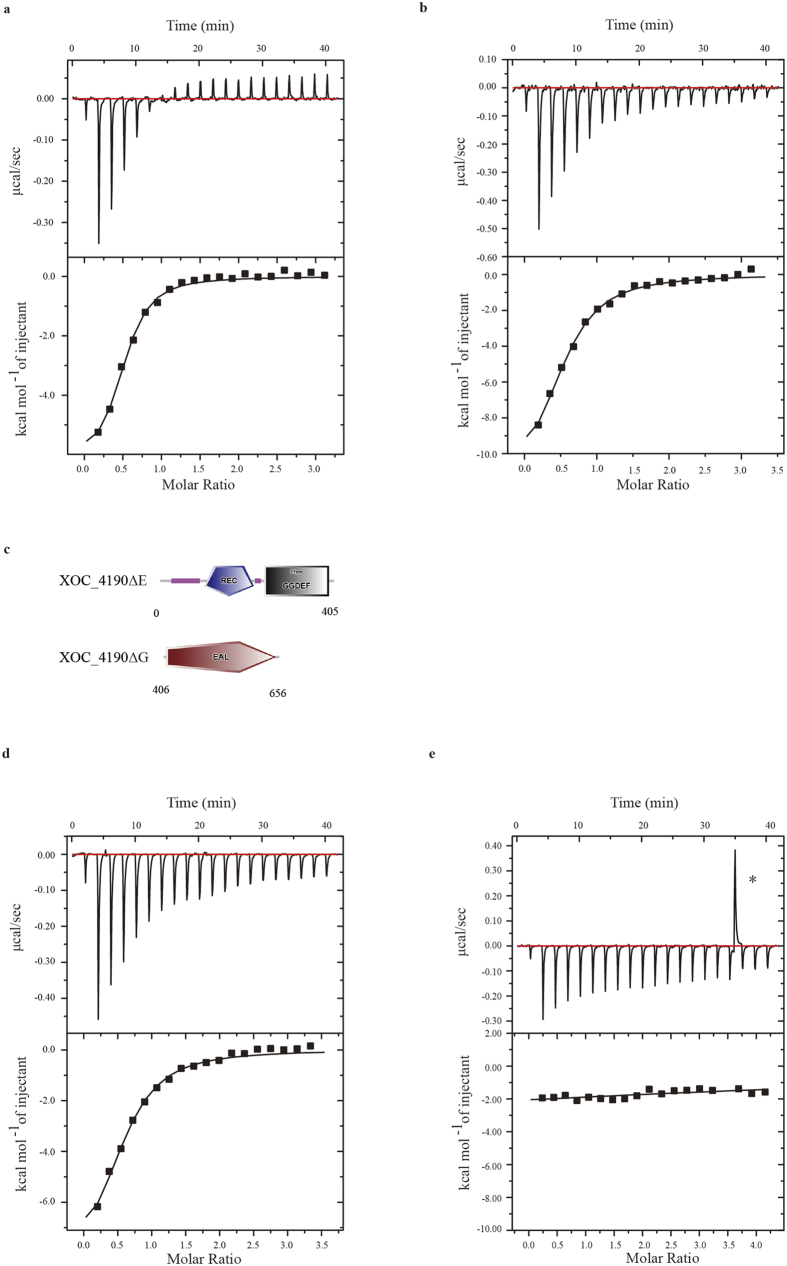
Figure 6. XOC_2102 and XOC_4190 bind to c-di-GMP in vitro.
Isothermal titration calorimetry (ITC) measurement for the interaction of c-di-GMP and XOC_2102 (a) or XOC_4190 (b). The data indicate that the dissociation constants (Kd) are 2.90 ± 0.13 μM or 4.59 ± 0.36 μM, respectively. (c) Schematic representation of the two truncated XOC_4190 proteins. XOC_4190ΔE, the truncated XOC_4190 without EAL domain; XOC_4190ΔG, the truncated XOC_4190 with the only EAL domain. ITC measurement for the interaction between c-di-GMP and XOC_4190ΔG (d) or XOC_4190ΔE (e). The data indicate that the Kd is 4.46 ± 0.28 μM for interaction between c-di-GMP and XOC_4190ΔG. Top panels, the titration calorimetry of the proteins with c-di-GMP at room temperature; lower panels, normalized ITC data for titrations versus molar ratio of c-di-GMP and the proteins.