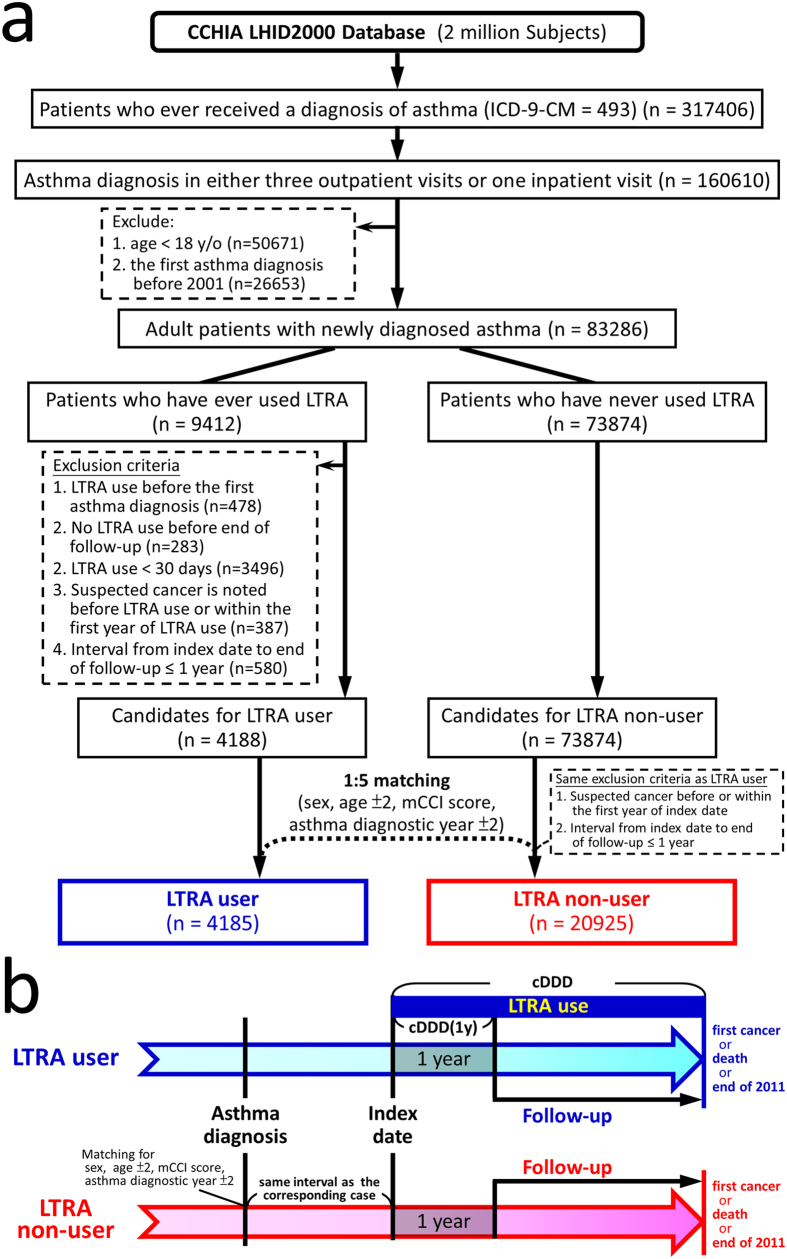
Figure 1.
(a) Algorism for identifying the study cohorts. (b) Study design. From the dataset, adult patients with newly diagnosed asthma were identified. Through the algorism, subjects using LTRA for more than a month (30 days) before the end of follow-up were identified as candidates for LTRA user cohort. The subjects who had never used LTRA were identified as candidates for LTRA non-user cohort. Each LTRA user was matched with five randomly-selected LTRA non-users by sex, age (±2), asthma diagnostic year (±2) and mCCI score. The index date was defined as the date of first LTRA prescription for LTRA users; the LTRA non-users were given the index date with the same interval from their first asthma diagnosis as their corresponding LTRA users. During the matching process, the same exclusion criteria for the LTRA users were also applied while selecting LTRA non-users to ensure enough follow-up time and absence of any cancer diagnosis before the end of the first year after index date. The subjects were followed from a year after the index date to either development of cancer, death or the end of 2011, whichever came first. The cumulative defined daily doses of LTRA were calculated from the index date to the end of follow-up (cDDD) and to a year after the index date [cDDD(1y)]. Abbreviations: CCHIA = Collaboration Center of Health Information Application; LHID = Longitudinal Health Insurance Database; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification code; mCCI = modified Charlson Comorbidity Index.