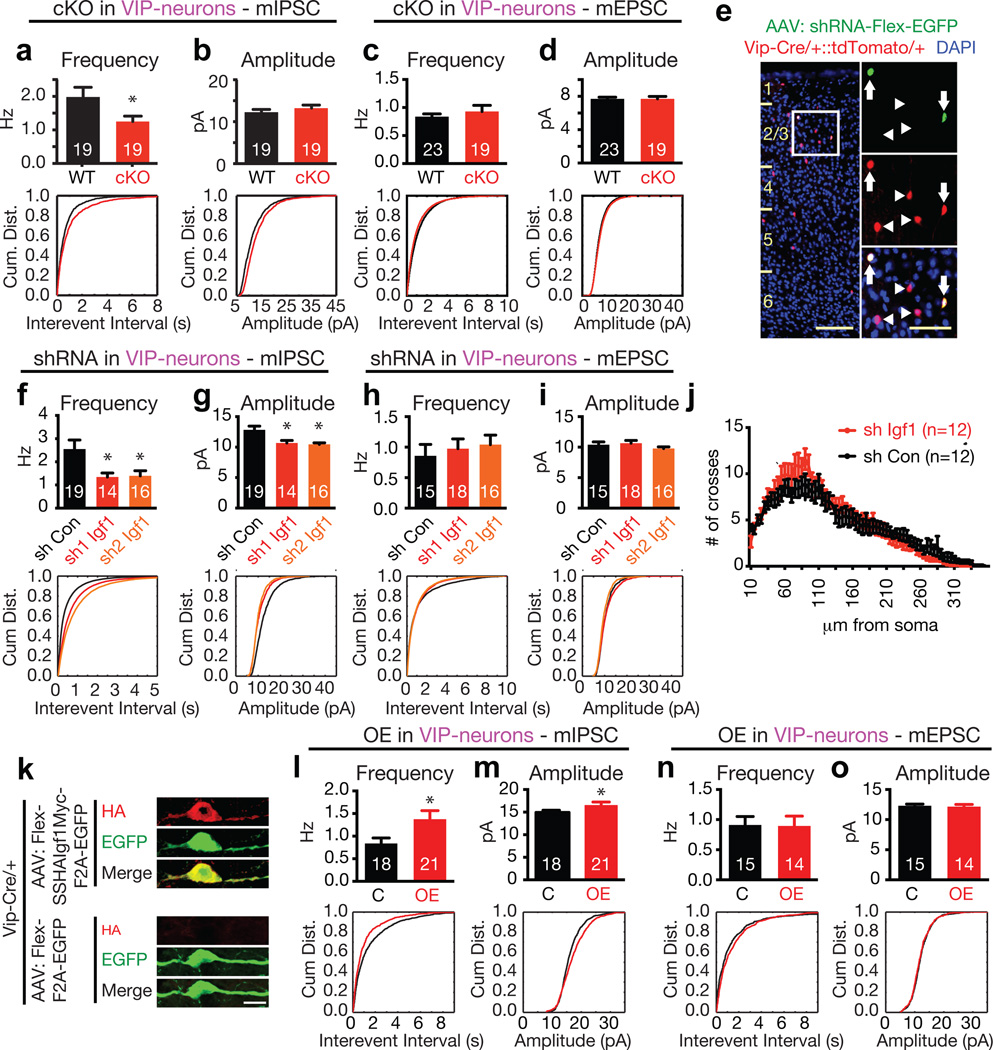
Figure 3. IGF-1 promotes inhibitory inputs to VIP neurons in a cell autonomous manner.
a–d) Bar graph and cumulative distribution of the frequency and interevent intervals of mIPSCs or mEPSCs recorded from Igf1 WT or cKO VIP neurons (mIPSC frequency: p = 0.046; amplitude p = 0.3. mEPSC frequency: p = 0.44; amplitude p = 0.9, Mann-Whitney U-Test).
e) Example image of sparsely infected VIP neurons upon injection of AAV-shRNA-hUbc-Flex-EGFP into mice expressing tdTomato in all VIP neurons (white box = magnified area; arrows = infected VIP neurons; arrowheads = non-infected VIP neurons; scale bars, 100 µm in main image, 50 µm in magnification)
f–i) Bar graph and cumulative distribution of mIPSC/mEPSC frequency, interevent interval and amplitude recorded from VIP neurons sparsely infected with control or Igf1 shRNAs (mIPSC Frequency: shRNA1 p = 0.05, shRNA2 p = 0.042. mIPSC Amplitude: shRNA1 p = 0.004, shRNA2 p = 0.001. mEPSC Frequency: shRNA1 p = 0.13, shRNA2 p = 0.07. mEPSC Amplitude: p = 0.77, shRNA2 p = 0.44. Mann-Whitney U-Test).
j) Sholl analyses of VIP neurons infected with control or Igf1 shRNA (p = 0.76, two-way repeated-measures ANOVA).
k) Expression of epitope-tagged Igf1.4 in VIP-neurons. Cortices of P3 Vip-Cre/+ mice were injected with AAVs driving Cre-dependent expression of SSHAIgf1.4Myc-F2A-EGFP (top) or F2A-EGFP (bottom) and stained at P20 for HA (red) and EGFP (green) (Scale-bar = 10 µm).
l–o) Bar graphs and cumulative distribution plots showing mIPSC/mEPSC amplitude and frequency / interevent interval in VIP neurons infected with control or IGF1-OE AAVs. (mIPSC: Amplitude, p = 0.05; Frequency, p = 0.02. mEPSC: Amplitude, p = 0.55; Frequency, p = 0.86. Mann Whitney U-Test)
a–d, f–j, l–o) Numbers inside bars indicate the number of cells recorded