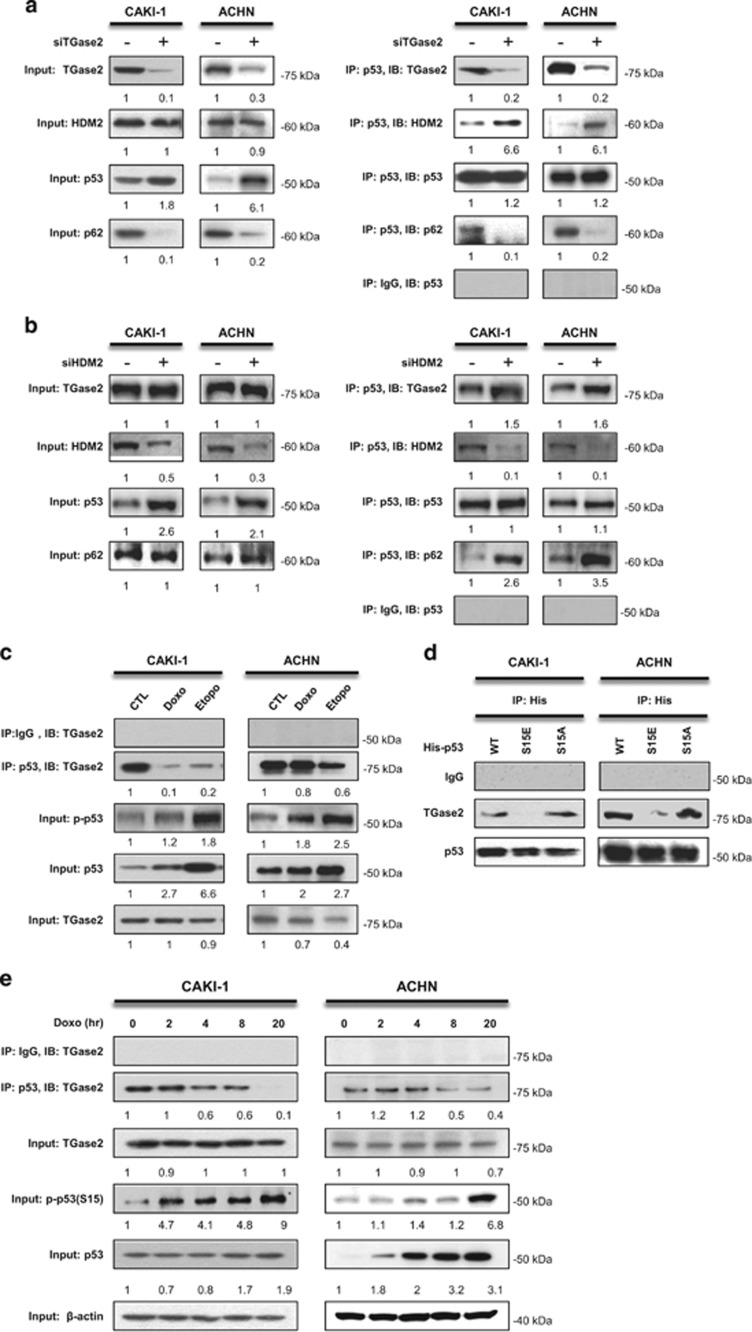
Figure 2.
TGase 2 and HDM2 compete for p53 interaction. TGM2 knockdown increased the interaction of p53 with HDM2, whereas it abolished the interaction with p62 (a and b). ACHN and CAKI-1 cells were transfected with siRNA for TGM2 (a) or HDM2 (b) for 48 h under starvation conditions. Whole-cell extracts (left) or p53 immunoprecipitates (right) were subjected to immunoblotting for TGase 2, HDM2, p53 and p62. (c) The induction of DNA damage inhibited the binding of TGase 2 to p53 and induced p53 phosphorylation. CAKI-1 and ACHN cells were treated with doxorubicin (1 μM) or etoposide (50 μM) for 24 h; whole-cell extracts were subjected to p53 immunoprecipitation and western blotting. (d) The activated p53 mimic (S15E) did not interact with TGase 2. CAKI-1 and ACHN were transfected with expression plasmids for p53 (wild type), p53 (S15E), or p53 (S15A) for 24 h, and the cell lysates were immunoprecipitated with a His-tag antibody and subjected to immunoblotting. (e) p53 stabilization (p-p53) by doxorubicin blocked interaction with TGase 2 in a time-dependent manner. After doxorubicin (1 μM) treatment for up to 20 h, cell lysates were immunoprecipitated with an anti-p53 antibody and subjected to immunoblotting. The blots are representative of three independent experiments