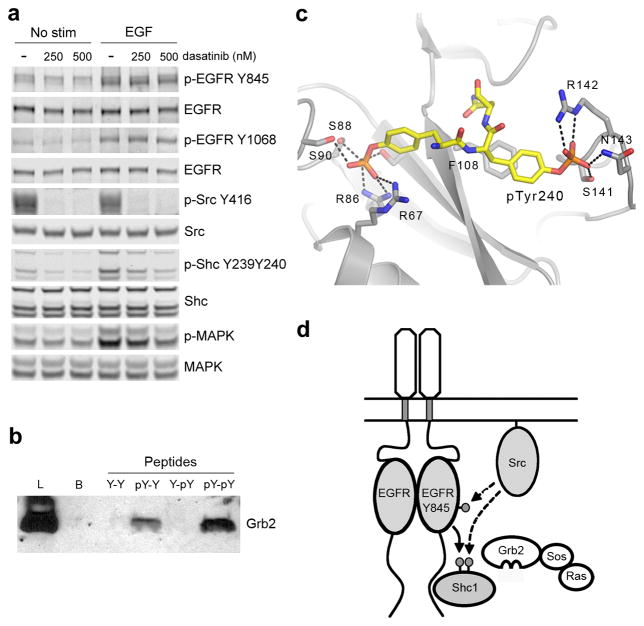
Figure 4.
Dual phosphorylation of Tyr239 and Tyr240 is controlled by EGFR and Src in cells and enhances Shc1 binding to Grb2. (a) Immunoblot analysis of cell lysates prepared from MCF10A cells pretreated with dasatinib for 1 hour then stimulated with 1ng/mL EGF for 3 min. (b) Pulldown assay and anti-Grb1 immunoblot. Samples are lysates from A431 cells incubated with the indicated biotinylated phosphopeptides. (Lysate = L; Control Beads = B; DHQYYNDFPGKE = Y-Y; DHQYpYNDFPGKE = Y-pY; DHQpYYNDFPGKE = pY-Y; DHQpYpYNDFPGKE = pY-pY). (c) Structure of a pseudo-peptide based on the sequence of Shc1 phosphorylated at Tyr239 and Tyr240 (mAZ-pY-(αME)pY-N-NH2) bound to the SH2 domain of Grb2 (PDB ID:1JYQ)31. Hydrogen bonds are indicated with dashed lines. (d) Model for the integration of EGFR and Src signaling by coordinated phosphorylation of Shc1. EGFR phosphorylation of Shc1 at Tyr239 creates a binding site for Grb2, leading to activation of the Ras-MAPK pathway. Active Src enhances activation by phosphorylating Tyr845 of the EGFR as well as Tyr240 of Shc1. Phosphorylation of Tyr240 of Shc1 potentiates phosphorylation at Tyr239 by the EGFR and enhances the binding of Grb2. Full-size images are shown in Supplementary Data Set 1.