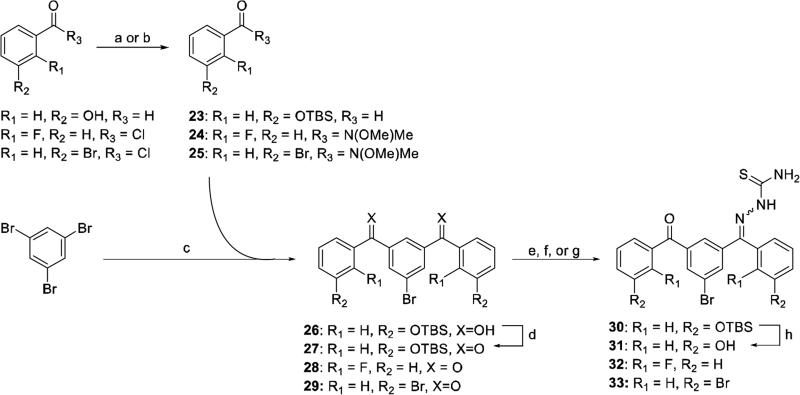
Scheme 4.
Synthesis of benzoylbenzophenone thiosemicarbazone analogues utilizing 1,3,5-tribromobenzene. Reagents and conditions: (a) Me(OMe)NH ·HCl, NEt3, CH2Cl2 0 °C −rt; (b) TBSCl, DMF, imidazole, 0 °C −rt; (c) (i) t-BuLi, ether, −78 °C; (ii) 23, 24, or 25 in ether, −78 °C; (d) PCC, celite, CH2Cl2, 0 °C −rt; (e) TSC, TsOH, THF, microwave irradiation, 90 °C; (f) TSC, TsOH, THF, reflux; (g) TSC, Ti(OiPr)4, THF, reflux (h) TBAF, THF, rt.