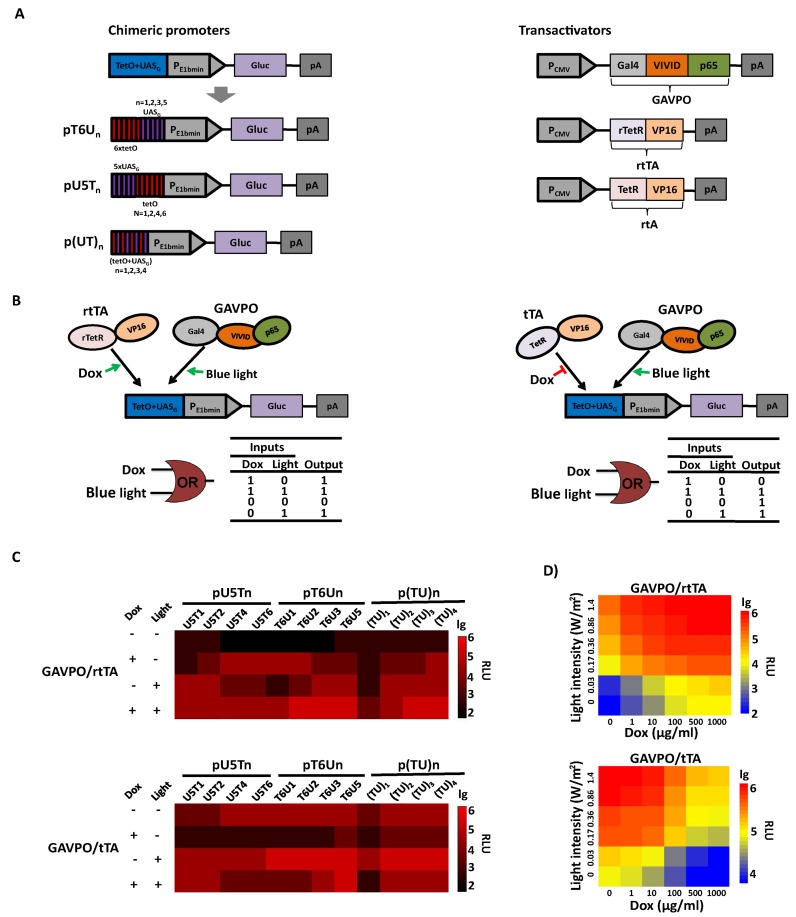
Figure 1.
Synergistic dual-input genetic circuits triggered by TET and light in mammalian cells. (A) Design of the synergistic controllers by GAVPO and tTA or rtTA triggered by TET and blue light. Three types of promoter configurations were constructed: (i) pU5Tn (n = 1, 2, 4, 6), six copies of tetO were placed at the 5′ end of different copies of the UASG element adjacent to the E1b minimal promoter; (ii) pT6Un (n = 1, 2, 3, 5), where five copies of the UASG element are placed at the 5′ end of different copies tetO adjacent to the minimal promoter and (iii) p(TU)n (n = 1, 2, 3, 4), the operator region comprises tetO alternating with UASG, and the copy number differs from one to four. The Gluc gene was used as the reporter gene and placed downstream of the E1b minimal promoter. Expression of the light-switchable transactivator GAVPO, Tc-responsive transactivators tTA and rtTA were all driven by the strong human cytomegalovirus (CMV) promoter. (B) Schematic representation of synergistic dual-input genetic circuits. Binding of the transactivator tTA and rtTA to the tetO in the absence and presence of Dox, respectively, or the binding of GAVPO to the UASG upon blue light exposure would result in gene activation. (C) Validation of the synergistic dual-input genetic circuits with different chimeric promoters. HEK293 cells were transiently co-transfected with a Tc-responsive transactivator plasmid (encoding tTA or rtTA), a light-switchable transactivator plasmid (encoding GAVPO), and a reporter plasmid (encoding Gluc under the control of different chimeric promoters). Gluc expression at different conditions was scored in the culture supernatant after 24 h. (D) Qualitative expression by modulating light irradiance and Dox concentration. The engineered cells were cultured at different light irradiances (0–1.4 W/m2) and Dox concentrations (0–1000 ng/ml). Gluc activity was determined after 24 h. The data in (C) and (D) were collected from three independent experiments.