Abstract
Purpose
Recently, we observed that telomeres of BRCA1/2 mutation carriers were shorter than those of controls or sporadic breast cancer patients, suggesting that mutations in these genes might be responsible for this event. Given the contradictory results reported in the literature, we tested whether other parameters, such as chemotherapy, could be modifying telomere-length.
Methods
We performed a cross-sectional study measuring leukocyte telomere-length of 266 sporadic breasts cancer patients treated with first-line chemotherapy, with a median follow up of 240 days.
Additionally, we performed both cross-sectional and longitudinal studies in a series of 236 familial breast cancer patients that included affected and non-affected BRCA1/2 mutation carriers. We have measured in leukocytes from peripheral blood: The telomere-length, percentage of short telomeres (<3Kb), telomerase activity levels and the annual telomere shortening speed.
Results
In sporadic cases we found that chemotherapy exerts a transient telomere shortening effect (around 2 years) that varies depending on the drug combination.
In familial cases, only patients receiving treatment were associated with telomere shortening but they recovered normal telomere-length after a period of two years.
Conclusion
Chemotherapy affects telomere-length and should be considered in the studies that correlate telomere-length with disease susceptibility.
Keywords: Chemotherapy, Sporadic Breast Cancer, Familial Breast and Ovarian Cancer, telomere-length, Telomerase
INTRODUCTION
Telomeres are nucleoprotein structures that cap and protect the ends of chromosomes against chromosomal fusion, recombination and terminal DNA degradation 1. In humans, the average telomere-length typically ranges from 10 to 15 kb 2, but telomere DNA shortens with each cell replication. This leads to a progressive telomere shortening during life that ranges from 24.7 to 45.5 bp/year 3. This shortening continues until the telomere reaches a critical length 4, which triggers cell-cycle arrest leading to senescence or apoptotic cell death.
Germ cells and stem cells can counteract telomere shortening through the action of an enzyme called telomerase, which can synthesize telomeric DNA de novo 5. However, cancer cells present altered DNA damage response mechanisms and are able to divide even when they present critically short telomeres by up regulating telomerase activity or activating the ‘alternative lengthening of telomeres’ mechanism 5.
There are several retrospective case-control and longitudinal studies suggesting that short telomeres found in DNA from surrogate tissues may predispose to different diseases including cancer 6-11. However, the largest prospective study in the general population to date (47,102 subjects with a 20-year follow-up) has recently suggested only a weak relationship between short telomeres and breast cancer risk among a large group of cancer types 12.
These results led to a contradiction in the literature regarding the association between telomere-length and cancer or other diseases, and point to differences in the strategies used to perform these studies (prospective or retrospective), technical issues, and/or the existence of telomere-length modifiers as sources of variability 13-16.
In a previous study we found that patients with hereditary breast cancer (BRCA1/2 and non-BRCA1/2 carriers) presented shorter telomeres than sporadic breast cancer patients and the control population, suggesting a modifier effect of the BRCA1 and BRCA2 genes on telomere-length17. Because our study was retrospective, like two other recent studies that presented contradictory results 18, 19, we decided to investigate the possible cause of these discrepancies.
We have focused our attention on the possible impact of chemotherapeutic cancer treatment, because some publications have already suggested a telomere shortening effect after the administration of chemotherapy in vivo (in different tumors) and in vitro (in normal human leukocytes and a mouse spermatogonial cell line) 20, 21.
We have explored this hypothesis in sporadic and familial breast cancer, and we have found that chemotherapy has a telomere shortening effect that is reversible after a period of time once the treatment is finished.
SAMPLES AND METHODS
Samples
Two different series of patients and two different strategies were used. We took a cross-sectional approach in a set of sporadic breast cancer cases and both a cross-sectional and a longitudinal approach in a series of familial breast cancer cases.
The first series comprised 253 sporadic breast cancer patients (age range: 30-81years) who were undergoing or had already received chemotherapy and 13 patients recently diagnosed that had not received chemotherapy (age range: 28-68y). They were included in a clinical trial that involved chemotherapy based on taxane derivatives and were followed for a median of 240 days (range 5-1855 days). We established two main categories based on their status: “During treatment” and “Post treatment”. In addition, patients were classified according to the taxane combination they received (Table1). Details regarding doses, number of cycles, duration and patient follow-up after treatment are summarized in Table S1.
Table 1.
Details regarding the two main Taxane subgroup schedules
| During treatment | After treatment | |||||
|---|---|---|---|---|---|---|
| Drug/Combination | n | Me-days treatment | Range/days * | n | Me-Days post treatment | Range/days # |
| AC+T/T+AC | 24 | 88 | 0-204 | 42 | 166.5 | 14-1855 |
| FEC+T/T+FEC | 63 | 85.5 | 0-161 | 124 | 281 | 1-1219 |
Me-days treatment: Median number of treatment days; Me-days post treatment: Median post treatment follows up in days; A: Doxorubicin; C: Cyclophosphamide; F: 5-Fluorouracil; E: Epirubicin; T: Paclitaxel
Days counted from the first day of treatment.
Days counted from last day of treatment.
The second series consisted of 236 familial breast cancer patients belonging to 104 families (23 harboring deleterious mutations in BRCA1, 28 in BRCA2, and 53 not associated to any of the known genes (BRCAX)). Most of the families were selected from the registry of the Familial Cancer Consultation of the CNIO and had been attended between 2011 and 2013. Individuals from these families met high risk criteria and 22 had been previously screened for mutations in BRCA1 and BRCA2 by a combination of denaturing high-performance liquid chromatography (DHPLC) and direct sequencing as previously reported 23. Due to the lack of a complete knowledge concerning the specific chemotherapy treatment regimens and the follow up of the patients after finishing the treatment, we established two general groups of patients. Only patients that were known to have been treated with any kind of chemotherapy were included in this analysis. Those, whose sample was extracted less than two years after diagnosis were considered as “During treatment”; while those whose sample was extracted more than two years after diagnosis were considered as “Post treatment”.
We measured leukocyte telomere-length by quantitative PCR, in the DNA of all individuals from this series, and whenever possible we measured the telomere-length and the percentage of short telomeres by High Throughput QFISH, and the telomerase activity levels in peripheral blood. In the FBOC series, we had the opportunity to compare 2 telomere-length measurements that corresponded to samples obtained at two different time points, with an average of 6 years between both extraction points. Using the 2 measurements we were able to calculate the telomere shortening rate per year.
A summary of all pertinent information (sample size, median age, age range and the experiments performed) of both cohorts of patients is shown in Table 2.
Table2.
General table with relevant information of the sporadic and familial breast cancer series
| n | Non Treated | Treated | Pos Treated | Age Median (y) | Age (Range) | Gap (years) | Telomere Length | Telomere Shortening Rate | Telomerase activity | High Throughput QFISH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls1 | 330 | 330 | - | - | 43 | 19-77y | + | - | - | ||
| Sporadic | 266 | 13 | 88 | 165 | 50 | 30-81y | - | + | - | - | - |
| Familial | 236 | 54 | 45 | 68 | 50 | 20-87y | - | + | - | + | + |
| Controls2 | 69/62 | - | - | - | 50 | 19-87y | - | - | - | + | + |
| Healthy-BRCA1/2 | 54 | 54 | - | - | 42 | 20-84y | - | + | - | + | + |
| Post treated-BRCA1/2 | 35 | - | - | 35 | 54 | 32-87y | - | + | - | + | + |
| Post treated-BRCAX | 33 | - | - | 33 | 51 | 32-72y | - | + | - | + | + |
| During treatment-BRCA1/2/X | 45 | - | * | - | 45 | 26-69y | - | + | - | + | + |
| Familial (2 time points) | 56 | 40 | 7 | 9 | - | - | 6 | - | + | - | - |
| Controls3 | 25 | 25 | - | - | 35 | 22-55y | 5 | - | + | - | - |
| Healthy-BRCA1/2 | 15 | 15 | - | - | 29 | 19-61y | 6 | - | + | - | - |
| Post treated-BRCA1/2 | 9 | - | - | 9 | 47 | 35-58y | 6 | - | + | - | - |
| During treatment-BRCA1/2 | 7 | - | 7 | - | 46 | 29-61y | 7 | - | + | - | - |
Controls1: Set of control used for obtain the adjustment line for the age; Controls2: Comprise the set of Healthy family members without mutations in BRCA1/2 used as controls for the Telomerase activity (n=69) and High Throughput QFISH (n=62), comparative analysis; Controls3: Controls that presented 2 samples, separated in time. These controls have been used to calculate the Telomere Shortening Rate of controls and to perform the comparative analysis;
From a total of 45 “During treatment-BRCA1/2/X”patients, 15 were BRCA1/2 and 30 BRCAX.
A set of 330 healthy Spanish women previously described 24, without personal or familial antecedents of cancer (age range 19-77y) was used as a control population (Table2-controls1) in order to adjust the telomere-length of both sporadic and familial breast cancer cases according to age.
The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to sample collection, and the study was approved by the Ethics Committee of Clinical Investigation from Centro Integral Oncologico Clara Campal, Madrid.
Telomere-length quantification
DNA from peripheral blood was extracted using MagNA Pure LC 2.0 System (Roche Diagnostics, Indianapolis, Indiana). Telomere-length was measured using quantitative PCR-based technique. This technique calculates the telomere-length as a ratio of telomere amount (t) relative to 36B4 reference gene amount (s)25.The measurement unit used in this technology is the t/s value. Primers used to perform the quantitative PCR have been described previously17. DNA samples were amplified in a total reaction volume of 10 μl containing 1× Gotaq quantitative PCR Master Mix (Promega, Madison, Wisconsin), 500 nM of primer Tel1, 1000 nM of primer Tel2, 10-30 ng of DNA and a common thermal cycling profile for both “t” and “s” determination. For 36B4 reactions, the concentrations of primers were 500 nM of 36B4u and 500 nM of 36B4d. All samples were analyzed in triplicate using an ABI 7900HT thermal cycler, in 384-well format. Telomere-length was calculated as previously described 17.
Measurement of telomere-length and short telomere content by High Throughput Q-FISH
For telomere-length by High Throughput Q-FISH measurement, peripheral blood mononuclear cells were hybridized with a PNA-tel Cy3-labeled probe. Telomere-length was determined as previously described 26 .
DAPI and Cy3 signals were acquired simultaneously in separate channels using a Leica TCS-SP5 confocal microscope (Wechsler, Germany). and maximum intensity projections from image stacks were generated for image quantification. In all cases, background noise was subtracted from the image prior to quantification. The “average telomere fluorescence” values represent the average Cy3 pixel intensity for the total nuclear area. Percentage of cells with short telomeres was calculated as those with intensity values within the first quartile.
Telomerase Assay
Protein extracts were obtained from peripheral blood mononuclear cells cultured in RPMI supplemented with 20 % fetal bovine serum and phytohemagglutinin during 4-5 days, according to the recommendations of the manufacturer of the TRAPeze telomerase detection kit (Merck Millipore, Darmstadt, Germany). The average telomerase activity was determined in each sample using 0.5, 0.25 and 0.125 μg of protein extract and normalized with the internal control included in the assay.
Telomere Shortening Rate
When possible, we calculated the difference in t/s values between blood samples extracted in two different time points (average of six years). The result was divided by the number of years that separated both samples. A positive result indicated shortening, while a negative value indicated elongation of the telomere.
Statistical analysis
Telomere-length measurements were adjusted for age using the line of best fit for controls. Thus, the difference between the actual and the predicted value was calculated for each sample. Following this method we adjusted t/s values obtained by quantitative PCR (y=−0.0174*X+1.96), the Kb obtained by High Throughput Q-FISH (−0.0587*X+12.007) and the percentage of short telomeres (0.1501*X+11.331).
The Kolmogorov-Smirnov test was used to evaluate whether telomere-length measurements obtained from healthy controls, familial breast cancer and sporadic cancer cases followed a normal distribution. As a normal distribution could not be assumed for healthy controls, a Mann-Whitney U test was applied (Table S2, figure 3a and figure 3b) Unpaired Student t-test for normal distributions was applied in the comparative analysis of telomerase activity, telomere shortening rate (Table S3 and Table S4) and percentage of short telomeres among the familial breast cancer groups (Figure 4).
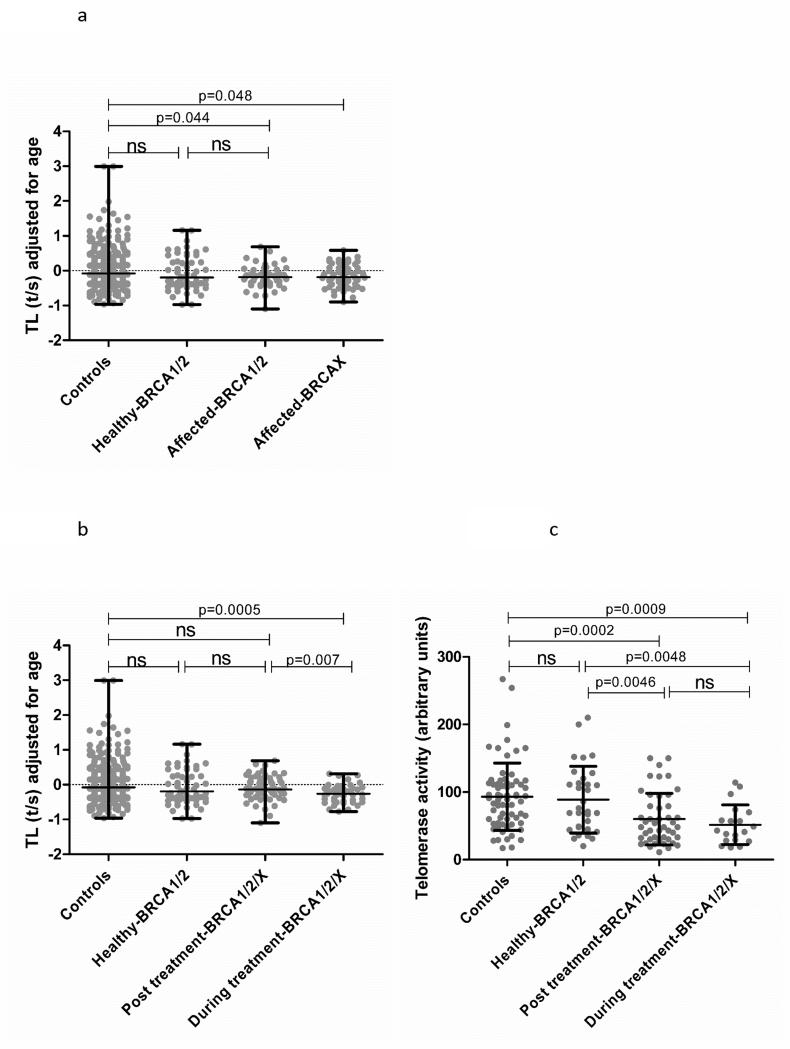
Figure 3.
a) Distribution of telomere-length (t/s) values adjusted for age for the familial breast cancer groups according to mutational status. The Mann Whitney test was used to test for significant differences in telomere-length between controls and the different subgroups. b) Distribution of telomere-length (t/s) values adjusted for age for the familial breast cancer groups according to treatment status (Mann Whitney test). c) Comparative analysis of telomerase activity levels in the familial breast cancer series; Student’s unpaired t-test was used to determine significant differences among groups.
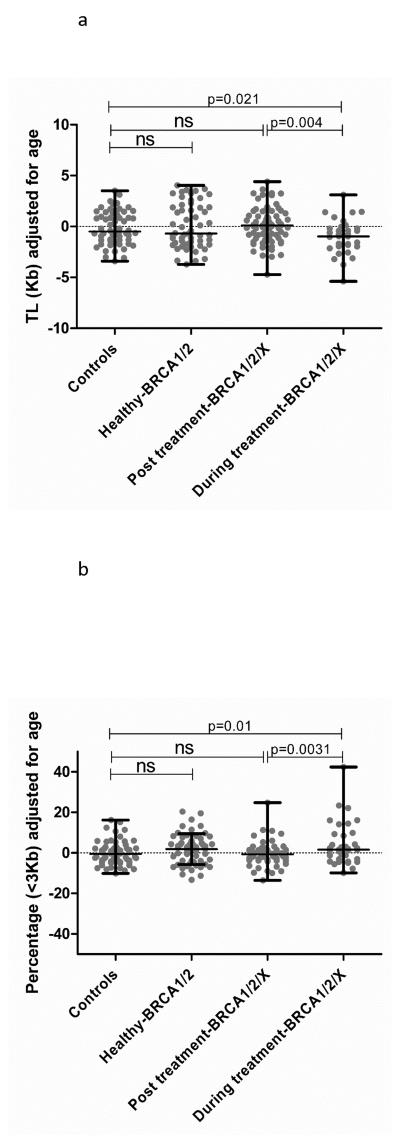
Figure 4.
a) Comparative analysis regarding telomere-length (adjusted Kb) in the familial breast cancer series (Unpaired Student t-test). b) Comparative analysis regarding content of short telomeres (<3Kb) in the familial breast cancer series (Unpaired Student t-test).
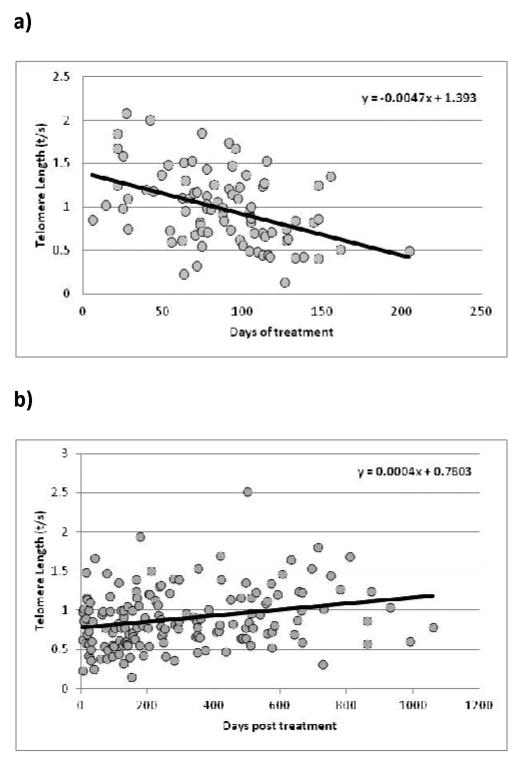
Spearman correlation test was used to establish whether correlation between variables were statistically significant (Figure 1).
Figure 1.
a) Correlation between telomere-length (t/s) and treatment time (r=−0.43; p=3.71E-05). b) Correlation between telomere-length (t/s) and time after treatment (r=0.17; p=0.02).
Statistical calculations were performed using SPSS version 18 (SPSS Inc, Chicago, Illinois) and Graph pad Prism 5.03(San Diego, California). Nominal two-sided p-values less than 0.05 were considered statistically significant. Graphics were performed using Graph pad Prism 5.03 and Microsoft Office Excel 2007.
RESULTS
Controls
We evaluated the telomere-length distribution in 330 healthy women as a function of age in order to obtain a regression line to adjust the t/s values from both the familial breast cancer and the sporadic series. As expected, we found a decrease in telomere-length with age. (Figure S1).
Sporadic cases
We tested whether chemotherapy itself has an effect on telomere shortening. To address this hypothesis we evaluated the telomere-length distribution of patients included in the “During treatment” category. We observed a strong correlation between telomere shortening and treatment duration (r=−0.43; p=3.71E-05) (Figure 1a). We did the same evaluation in the “Post treatment” category, and we observed that the distribution had the opposite shape, with a significant positive correlation between telomere-length and time after treatment (r=0.17; p=0.02) (Figure 1b).
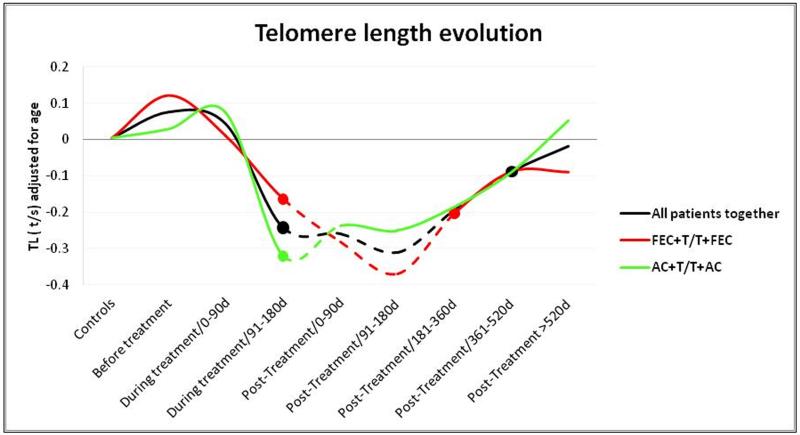
We next did a comparative analysis of telomere-length during different periods of the treatment time line, (Figure 2). We compared the median telomere-length of each group, with the median telomere-length of the control group. The telomere-length (t/s values adjusted by age) in patients before treatment did not differ compared to controls (Figure 2, black line). From the beginning of the treatment, we observed a shortening effect that became statistically significant in patients treated for more than 90 days and remained significantly shortened until 360 days after the treatment. Patients whose samples were extracted later than 360 days after the end of the treatment tended to recover normal telomere-length values, and did not show significant differences when compared to controls. The results of this analysis are summarized in Table S2.
Figure 2.
Telomere-length evolution during treatment and after treatment. Mann Whitney test was used to determine significant differences between controls and the different subgroups. The discontinuous lines represent significantly shortened telomeres compared to controls for all the patients together (black line) and for the two major drug schedules (red and green lines).
In patients treated with therapy based in taxane derivatives (Table 1), we observed a more significant telomere shortening effect in those treated with FEC+T/T+FEC (red line) compared to those treated with AC+T/T+AC (green line) however, the latter seemed to recover their telomere-length faster (Figure 2).
Familial Breast Cancer Cases
Cross-sectional study
We performed this analysis to explore both, the role of BRCA1/2 mutations as telomere-length modifiers and the possible correlation between telomere shortening and cancer treatment. Healthy BRCA1/2 mutation carriers (n=54) did not present shorter telomeres when compared to controls (n=330), while significantly shorter telomeres were found in affected BRCA1/2 mutation carriers (n=50; p=0.044) and BRCAX patients (n=63; p=0.048) (Figure 3a).
In view of the behavior of telomeres in sporadic breast cancer cases according to the treatment status, we decided to reanalyze the data from the familial breast cancer series. We established two categories consisting of cancer patients from whom samples were extracted within two years from the beginning of the treatment, called “During treatment BRCA1/2/X”, and a second group composed of patients whose samples were taken more than two years after the beginning of treatment, called “Post treatment-BRCA1/2/X”. We used two years as a cut-off, because in the sporadic series we observed that the recovery phase starts approximately two years since diagnosis.
No significant differences were found between “Post treatment-BRCA1/2/X” patients and controls (Table2-controls1). However, the “During treatment-BRCA1/2/X” group presented significant shortened telomere t/s values compared with the controls (p=0.0005) and the “Post treatment-BRCA1/2/X” groups (p=0.007) (Figure 3b).
We also evaluated the leukocyte telomerase activity in this cohort of patients (Figure 3c). We found that healthy BRCA1/2 mutation carriers presented similar telomerase activities to the healthy controls (Table 2-controls2). Interestingly, all cancer patients, independently of the mutation type and the treatment time, presented significantly lower levels of telomerase activity, being patients “During treatment” the ones showing the lowest values (p=0.0009) (Table S3).
In parallel, we used High Throughput Q-FISH technology in order to validate our results using an alternative technology. We found the same results regarding telomere-length for the “During treatment-BRCA1/2/X” group (Figure 4a), confirming the presence of a significantly higher percentage of short telomeres (<3Kb) for this group of patients (p=0.01) compared to the control population (Table 2-controls2) (Figure 4b).
Longitudinal study
We performed a longitudinal retrospective study in order to analyze telomere shortening rate in a set of 31 BRCA1/BRCA2 mutation carriers with two independent blood samples taken separately by an average period of 6 years: 15 were healthy carriers, 9 affected carriers with the two samples obtained once the chemotherapy had finished, and 7 affected carriers in which the first sample was obtained “During treatment” and the second extraction point in the “Post treatment”. Additionally we analyzed the telomere shortening rate in a set of 25 controls from which we also had samples taken at two time point (Table 2-controls3).
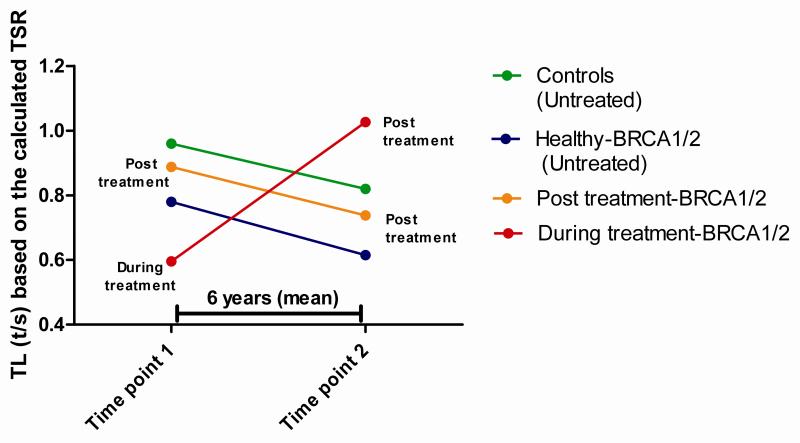
Healthy BRCA1/2 carriers presented a faster telomere shortening rate compared to the control group, although the differences were not significant; while “Post treatment-BRCA1/2” patients presented a telomere shortening rate very similar to the control group. By contrast, “During treatment-BRCA1/2” patients presented a different pattern, going from short telomeres at the first measurement, to normal telomeres 7 years later (Figure 5). In this case the elongation speed was very pronounced, and statistically different from the normal shortening of the control population (p=0.011). The results of this analysis are summarized in Table S4.
Figure 5.
Theoretical telomere-length (TL) evolution based on two telomere-length measurements along the time (average 6 years). We observed a similar behavior for controls, Healthy BRCA1/2 carriers and Post treatment affected patients carrying BRCA1/2 mutations regarding telomere shortening rate, ranging from 0.028 to 0.033 t/s per year. Strikingly patients with control samples during treatment and post treatment presented an opposite elongation pattern, of −0.086 t/s year.
DISCUSSION
We previously reported that BRCA1/2 might be telomere-length modifiers, because mutation carrier patients presented shorter telomeres than the control population and sporadic breast cancer cases 17. However, two recent reports did not find any relationship between the presence of BRCA1/2 mutations and telomere shortening, nor between telomere-length and an increased cancer risk 18, 19. The three studies were done in a retrospective manner, and in none of them treatment status, which might constitute a confounding factor, was evaluated independently as a possible modifier of telomere-length. We tried to overcome this possible bias by working with two independent cohorts of women with breast cancer (sporadic and familial) and two different approaches (cross-sectional and longitudinal).
According to the results obtained in the sporadic set of patients treated with different chemotherapy schedules and followed for a maximum period of 1855 days, telomere shortening starts at 91-180 days after the beginning of treatment and reaches its maximum effect between 91-180 days after finishing treatment. Then a recovery phase starts, and it lasts for a maximum of 1855 days after which the original telomere-length is recovered (Table S2). Similar results were observed in the familial breast cancer series in both, cross-sectional and longitudinal studies.
There are some reports suggesting a possible deleterious effect of chemotherapy on telomere-length and/or telomerase activity, although some of these seem to be contradictory.
The first authors, who addressed this question, reported significant telomere shortening accompanied by a significant reduction in telomerase activity in the blood cells of pediatric leukemia patients after the administration of cancer treatment 27. Another early longitudinal study in breast cancer patients undergoing chemotherapy, reported a clear negative effect on different hematological parameters, including telomere shortening, after high doses of FEC+T 28. On the other hand, in a recent longitudinal study performed in a set of 33 breast cancer patients undergoing AC+T/T+AC chemotherapy no changes were detected in telomere-length, at three and twelve months after the end treatment 29 .
It is probable that in this case patients were already at the “recovery phase” proposed in this study. Surprisingly, increased expression of the senescence markers p16 and ARF was detected, which have been correlated with telomere damage in primary cells 30.
Engelhard et al. were the first to raise the question of whether telomere shortening is permanent or telomeres regain their initial length after discontinuation of chemotherapy 27.
In this regard, our results confirm there is a telomere recovery after treatment, although we have observed that the shortening effect and the time to reach the “recovery phase” varies depending on the treatment type, with a shortening effect more severe at the initial phase of the treatment for patients under the AC+T/T+AC regimen, while those treated with FEC+T/T+FEC present a more delayed “recovery phase”. It seems that telomere stability is affected by conventional chemotherapies in different manner depending on the specific drug mechanism of action (Alkylating agents, anti-replicative molecules, spindle poisons, anti-topoisomerase I and II) being the effects on the telomere heterogeneous.
Some authors have evaluated the role of several chemotherapy drugs in vitro and in cancer patients at a more mechanistic level 21, 31, 32. For instance, the alkylating agent cyclophosphamide, was shown to induce in spermatogonial cells a significant increase in DNA damage, which was localized preferentially at telomeres. This telomere DNA damage was accompanied by telomere shortening and a significant reduction in both telomerase mRNA levels and significantly diminished telomerase activity 21. These negative effects may be explained due to the high guanine content of the telomere sequence and the TERC gene, which may increase the risk of DNA-DNA crosslink damage by cyclophosphamide 21.
In another longitudinal study using 6 cycles of CHOP (cyclophosphamide, doxorrubicine, vincristine and Prednisone) in a cohort of 14 Non-Hodgkin lymphoma patients, significant telomere shortening was maintained up to 2 years, after the treatment ends. The same study evaluated the anti-metabolite drug 5-fluoracil alone in a set of 10 colon cancer cases. TL was measured 2 and 12 months after the end of the treatment showing an initial telomere attrition that was recovered after one year 31. The authors propose a possible mechanism based on whether these drugs are able to target hematopoietic stem cells, or only mature progenitors, to explain a long or a short-term effect in telomere shortening 31. In addition, it is also known that mitotic inhibitors such as taxol, which are normally included in conventional breast cancer chemotherapy schedules, can causes telomere uncapping, triggering telomere dysfunction in cancer cells 32.
Taking all this evidences together we can assume some variation in the telomere dynamics during and after the treatment, depending which type of anticancer chemotherapy drugs are included in the patients treatment.
In the familial breast cancer model we followed both cross-sectional and longitudinal approaches in order to test whether the BRCA genes and chemotherapy are modifiers of telomere-length and/or telomerase activity.
In the cross-sectional approach we found similar results as in our previous study 17, familial cases presented shorter telomeres than controls; however, a substantial proportion of the samples had been taken during or after a short time of chemotherapy administration. When we corrected for treatment status we were not able to detect any effect of the BRCA1/2 mutations on telomere shortening, neither in healthy carriers nor in post treated-BRCA1/2 patients, suggesting that the treatment is the real telomere-length modifier (Figure 3b). These results could explain partially, the discrepancies reported in the literature regarding the role of the BRCA1/2 genes as modifiers of telomere-length17. In a recent report by Pooley et al, BRCA1/2 mutation carriers showed longer telomeres than controls, and the authors suggests that an increase in telomerase levels could be associated with this event 18. However, in the present study we have not found higher levels of telomerase activity in healthy BRCA1/2 patients, while treated patients (independently of their mutational status), presented both shorter telomeres and significant lower telomerase activity levels.
Regarding the longitudinal study, BRCA1/2 affected carriers with two telomere-length measurements covering the “Post treatment” period, did not present significant differences in the shortening rate compared to healthy BRCA1/2 carriers and/or controls. However, BRCA1/2 patients, with the first sample taken during treatment and the second sample taken 7 years later (during the post treatment period) presented a recovery of telomere-length after chemotherapy, confirming our observations in the sporadic series (Fig 5).
To summarize, our study has focused on the effect of treatment on telomere-length in both familial and sporadic breast cancer cases. We cannot rule out that BRCA1 and BRCA2 might be minor modifiers of telomere-length, but it appears that treatment is the true cause of telomere shortening. The rates of telomere shortening and recovery may vary depending on the treatment. These results stress the need to perform prospective and retrospective studies, considering the variability found as a consequence of the treatment status (untreated, during treatment and post treatment) of the patients, to obtain conclusive results about the relationship between telomere-length and disease.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Alicia Barroso and Victoria Fernandez for their technical support. This work was partially funded by project FIS PI12/00070 from the Carlos III Health Institute and the Spanish Network on Rare Diseases (CIBERER). By project SAF2012–35779 (Spanish Ministry of Economy and Competiveness). M.A.B.’s laboratory is funded with the Spanish Ministry of Science and Innovation, projects SAF2008-05384 and 2007-A-200950 (TELOMARKER), European Research Council Advanced grant GA#232854, the Körber Foundation, Botín Fundation, and Lilly Fundation.
Footnotes
Ethical standards:
The authors declare that this work complies with current Spanish laws.
Conflict of interest:
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Blackburn EH. Switching and signaling at the telomere. Cell. 2001 Sep 21;106(6):661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 2.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005 Aug;6(8):611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 3.Muezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. Mar;12(2):509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 5.Kong CM, Lee XW, Wang X. Telomere shortening in human diseases. FEBS J. Jul;280(14):3180–3193. doi: 10.1111/febs.12326. [DOI] [PubMed] [Google Scholar]
- 6.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. Jun;20(6):1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fyhrquist F, Silventoinen K, Saijonmaa O, et al. Telomere length and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. J Hum Hypertens. Dec;25(12):711–718. doi: 10.1038/jhh.2011.57. [DOI] [PubMed] [Google Scholar]
- 8.Willeit P, Willeit J, Kloss-Brandstatter A, Kronenberg F, Kiechl S. Fifteen-year follow-up of association between telomere length and incident cancer and cancer mortality. JAMA. Jul 6;306(1):42–44. doi: 10.1001/jama.2011.901. [DOI] [PubMed] [Google Scholar]
- 9.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. Jul 7;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 10.Shao L, Wood CG, Zhang D, et al. Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J Urol. 2007 Oct;178(4 Pt 1):1492–1496. doi: 10.1016/j.juro.2007.05.112. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003 Aug 20;95(16):1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 12.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. Apr 3;105(7):459–468. doi: 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- 13.Savage SA, Gadalla SM, Chanock SJ. The long and short of telomeres and cancer association studies. J Natl Cancer Inst. Apr 3;105(7):448–449. doi: 10.1093/jnci/djt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham JM, Johnson RA, Litzelman K, et al. Telomere length varies by DNA extraction method: implications for epidemiologic research. Cancer Epidemiol Biomarkers Prev. Nov;22(11):2047–2054. doi: 10.1158/1055-9965.EPI-13-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. Nov 1;39(20):e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006 Dec;35(6):1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Delgado B, Yanowsky K, Inglada-Perez L, et al. Genetic anticipation is associated with telomere shortening in hereditary breast cancer. PLoS Genet. Jul;7(7):e1002182. doi: 10.1371/journal.pgen.1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pooley KA, McGuffog L, Barrowdale D, et al. Lymphocyte telomere length is longer in BRCA1 and BRCA2 mutation carriers but does not affect subsequent cancer risk. Cancer Epidemiol Biomarkers Prev. Mar 18; doi: 10.1158/1055-9965.EPI-13-0635-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killick E, Tymrakiewicz M, Cieza-Borrella C, et al. Telomere length shows no association with BRCA1 and BRCA2 mutation status. PLoS One. 9(1):e86659. doi: 10.1371/journal.pone.0086659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Hou M, Lou F, Bjorkholm M, Xu D. Telomere dysfunction induced by chemotherapeutic agents and radiation in normal human cells. Int J Biochem Cell Biol. Sep;44(9):1531–1540. doi: 10.1016/j.biocel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, Hales BF, Robaire B. Effects of four chemotherapeutic agents, bleomycin, Etoposide, Cisplatin, and cyclophosphamide, on DNA damage and telomeres in a mouse spermatogonial cell line. Biol Reprod. 90(4):72. doi: 10.1095/biolreprod.114.117754. [DOI] [PubMed] [Google Scholar]
- 22.Milne RL, Osorio A, Cajal TR, et al. The average cumulative risks of breast and ovarian cancer for carriers of mutations in BRCA1 and BRCA2 attending genetic counseling units in Spain. Clin Cancer Res. 2008 May 1;14(9):2861–2869. doi: 10.1158/1078-0432.CCR-07-4436. [DOI] [PubMed] [Google Scholar]
- 23.Diez O, Osorio A, Duran M, et al. Analysis of BRCA1 and BRCA2 genes in Spanish breast/ovarian cancer patients: a high proportion of mutations unique to Spain and evidence of founder effects. Hum Mutat. 2003 Oct;22(4):301–312. doi: 10.1002/humu.10260. [DOI] [PubMed] [Google Scholar]
- 24.Osorio A, Endt D, Fernandez F, et al. Predominance of pathogenic missense variants in the RAD51C gene occurring in breast and ovarian cancer families. Hum Mol Genet. Jul 1;21(13):2889–2898. doi: 10.1093/hmg/dds115. [DOI] [PubMed] [Google Scholar]
- 25.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002 May 15;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canela A, Vera E, Klatt P, Blasco MA. High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5300–5305. doi: 10.1073/pnas.0609367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelhardt M, Ozkaynak MF, Drullinsky P, et al. Telomerase activity and telomere length in pediatric patients with malignancies undergoing chemotherapy. Leukemia. 1998 Jan;12(1):13–24. doi: 10.1038/sj.leu.2400889. [DOI] [PubMed] [Google Scholar]
- 28.Schroder CP, Wisman GB, de Jong S, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer. 2001 May 18;84(10):1348–1353. doi: 10.1054/bjoc.2001.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. Apr;106(4):dju057. doi: 10.1093/jnci/dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs JJ, de Lange T. p16INK4a as a second effector of the telomere damage pathway. Cell Cycle. 2005 Oct;4(10):1364–1368. doi: 10.4161/cc.4.10.2104. [DOI] [PubMed] [Google Scholar]
- 31.Diker-Cohen T, Uziel O, Szyper-Kravitz M, Shapira H, Natur A, Lahav M. The effect of chemotherapy on telomere dynamics: clinical results and possible mechanisms. Leuk Lymphoma. Sep;54(9):2023–2029. doi: 10.3109/10428194.2012.757765. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, Leong W, Guerin O, Gilson E, Ye J. Telomeric impact of conventional chemotherapy. Front Med. Dec;7(4):411–417. doi: 10.1007/s11684-013-0293-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.