Fig. 4. Reversal of immune tolerance by neutralizing HBs: a new strategy.
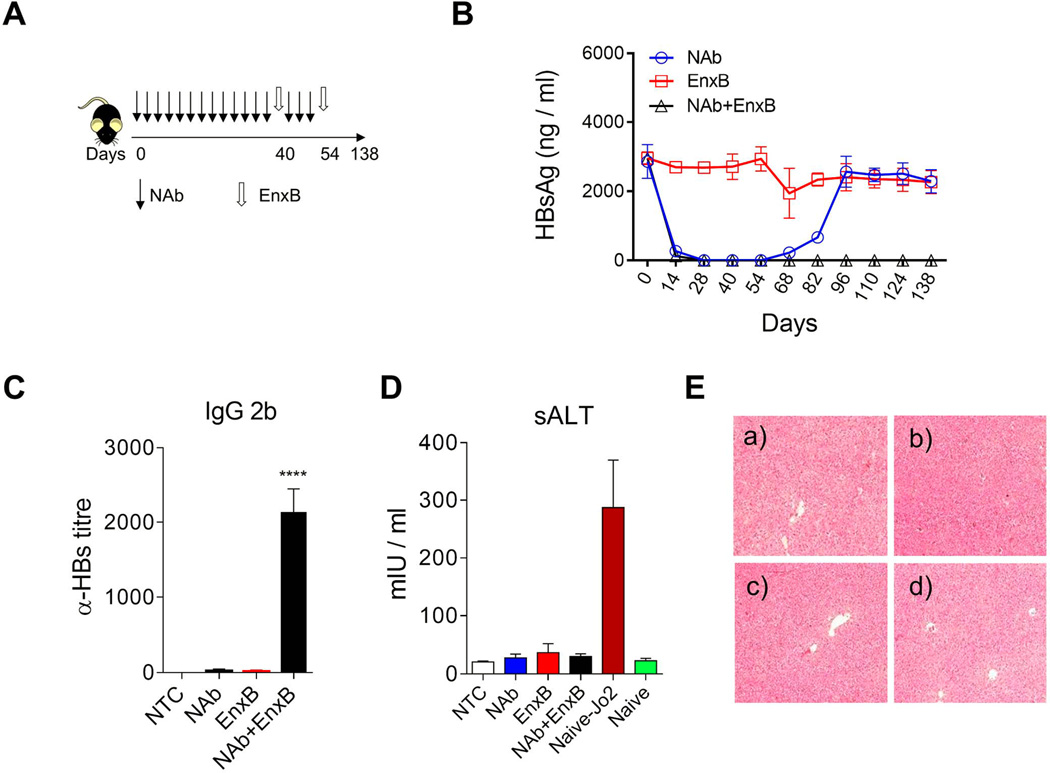
(A) Schematic treatment schedule of tolerant HBV carrier mice with a combination of HBs-neutralizing antibody (Ab-H, 1 mg/mouse with 3-days intervals) and vaccine (EnxB, 2 µg). (B) Serum levels of HBs in different treatment groups were monitored by ELISA (n=3). (C) De novo anti-HBs generation was evaluated on day 21 after the 2nd EnxB vaccination, by monitoring IgG subtype-2b antibodies via ELISA using ayw-HBs antigen identical to the HBV serotype in animal model (n=3). (D) Serum level of ALT was monitored on day 21 after the previous vaccination. Sera from Jo2 (a Fas agonistic antibody) treated B6 mice were used as the positive control (n=3). (E) HE staining for pathologic analysis of liver injury or inflammation. a) No treatment control, b) NAb treated group, c) EnxB treated group, d) combination treatment with NAb plus EnxB group. Experiments were repeated at least three times. Data shown here is one representative of three independent experiments.
