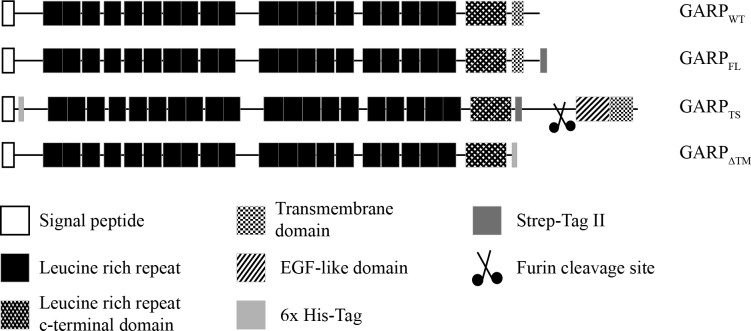
Fig 1. Domain structure of GARP and its recombinant variants.
Schematic representation of human GARP and its recombinant variants used in this study. GARP consists of a signal peptide, 20 leucine rich repeats, a leucine rich repeat C-terminal flanking domain and a transmembrane region. For the construct GARPFL a Strep-tag was added at the intracellular C-terminus. Instead of the original GARP transmembrane region, the construct GARPTS possesses the transmembrane region of the protease meprin α and additionally its extracellular EGF-like and inserted domain. This construct was cleaved by furin in the trans-Golgi network and secreted into the extracellular space. For purification and detection a Strep-tag was inserted between the extracellular part of GARP and the meprin α part and a His-tag between the signal peptide and the mature chain. GARPΔTM lacks the complete transmembrane region of GARP, but contains a His-tag instead at the C-terminus of the extracellular part.