Figure 3.
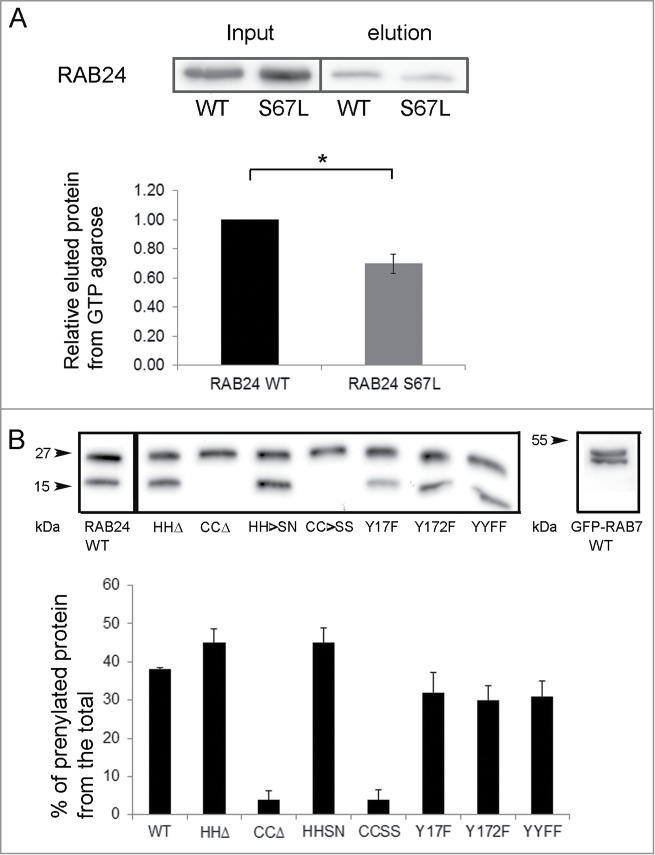
Analysis of RAB24 mutants. (A) GTP binding of RAB24S67L mutant was tested using GTP-agarose beads. Recombinant proteins were produced in E. Coli production strain BL21 and lysates were incubated with GTP-agarose gel. After washing, the bound protein was eluted from the gel with excess GTP, and western blotting was used to compare the eluted amounts of RAB24-WT and S67L. Eluted protein bands were normalized with their input bands. The columns and error bars show the mean and SEM from 4 independent experiments. (B) HeLa cells were transiently transfected with MYC-tagged wild-type or mutant MYC-RAB24 plasmids. Cell extracts were electrophoresed on urea (4 to 8 M) acrylamide (10–20%) SDS gradient gels and blotted on PVDF membranes. MYC antibody was used for detection. Prenylation-deficient mutants, CCΔ and CC→SS, lack the lower band, which represents the prenylated form of the protein. Nearly 40% of wild-type RAB24, and 45% of prenylation-competent mutants, are in the prenylated form. Tyrosine phosphorylation deficiency had a slight but nonsignificant effect on prenylation. The columns and error bars show the mean and SEM from 4 or 5 independent experiments. GFP-RAB7 is shown as a reference.
