Abstract
The Hippo signaling pathway controls organ size by orchestrating cell proliferation and apoptosis. When the Hippo pathway was inactivated, the transcriptional co-activator Yorkie translocates into the nucleus and forms a complex with transcription factor Scalloped to promote the expression of Hippo pathway target genes. Therefore, the nuclear translocation of Yorkie is a critical step in Hippo signaling. Here, we provide evidence that the N-terminal 1–55 amino acids of Yorkie, especially Arg-15, were essential for its nuclear localization. By mass spectrometry and biochemical analyses, we found that Importin α1 can directly interact with the Yorkie N terminus and drive Yorkie into the nucleus. Further experiments show that the upstream component Hippo can inhibit Importin α1-mediated Yorkie nuclear import. Taken together, we identified a potential nuclear localization signal at the N-terminal end of Yorkie as well as a critical role for Importin α1 in Yorkie nuclear import.
Keywords: Hippo pathway, nuclear translocation, nuclear transport, oncogene, yes-associated protein (YAP), Importin, NLS, yorkie
Introduction
The mechanisms by which organ size is controlled during development have long been an intriguing question. Extrinsic environmental factors and intrinsic signals function cooperatively in organ development (1). The Hippo pathway, initially discovered in Drosophila by genetic screens, restricts organ size through inhibiting cell growth and promoting cell death (2, 3). In Drosophila, the core to the pathway is a kinase cassette including the kinases Hippo and Warts, which trigger the phosphorylation of the transcription coactivator Yorkie (Yki)3 (4–9). In the absence of suppression from Hippo signaling, Yki translocates into nuclei and hence associates with transcription factor Scalloped (Sd) (10–12). As a result, the Yki-Sd complex induces the expression of genes, including diap1 (death-associated inhibitor of apoptosis 1), cyclin E, and bantam microRNA, to regulate cell proliferation and apoptosis (13–15).
The core components of the Drosophila Hippo pathway are highly conserved in mammals. Although the regulation of cytoplasm-to-nucleus translocation of Yki or its mammalian homologue YAP (Yes-associated protein) is a fundamental step in Hippo signaling, little is known about the underlying mechanisms. The nuclear localization of proteins is mediated by two mechanisms. One is passive diffusion through the nuclear pore complex (NPC), which is only suitable for proteins smaller than ∼40–50 kDa (16, 17). The second one is an energy-dependent process, which allows proteins that contain a nuclear localization signal (NLS) to enter the nucleus (18, 19). NLSs are short peptide motifs that mediate the nuclear import of proteins. Classical NLSs always have one or two clusters of basic amino acid residues, and two clusters always separated by a 10–12 amino acid linker, defined as (K/R)(K/R)X10–12(K/R)3/5 (20–23). However, a number of experimentally defined NLSs do not match the consensus sequences; sometimes they contain only one or two lysines or arginines (24–26).
In mammalian systems, NLSs are usually recognized by the heterodimeric receptor proteins Importin α/β. Importin-substrate complexes translocate into the nucleus through the NPC, and with the help of RanGTP/RanGDP, the Importin α can recycle back to the cytoplasm (27, 28). Crystallographic approaches and mutational analysis reveals that the cNLS-binding domain of Importin α is located within a shallow groove on the concave face, which is comprised of ten armadillo (ARM) repeats. The primary binding site for NLSs spans ARM repeats 1–4 (the major site), and a secondary site (the minor site) spans ARM repeats 6–8 (29–32). In Drosophila melanogaster, the Importin α family contains Importin α1, Importin α2, and Importin α3 (33–35). Importin α1 and α2 play distinct roles in male and female gametogenesis (36, 37); Importin α3 is required for larval survival, structure development, and completion of oogenesis (35).
In this study, we demonstrate that the N-terminal 1–55 amino acids of Yki may serve as its NLS and is essential for Yki function and localization. We also identify Importin α1 as a mediator of Yki nuclear translocation using mass spectrometry analysis. Furthermore, we show that the amino acid Arg-15 of Yki is a critical site for Yki nuclear import, which is regulated by upstream core kinase Hippo. Therefore, our observations demonstrate the functional and physical interactions between Yki and Importin α1.
Materials and Methods
Cloning, Drosophila Stocks, and Genetics
Importin α1, α2, α3, β cDNAs were amplified by PCR and introduced into pUAST-HA or pUAST-Flag vector. Yki point mutations and deletions were generated by PCR-based site-directed mutation. The YkiN mutant form was produced by gene synthesis (Generay). All plasmids were verified by DNA sequencing.
Transgenic flies expressing α1 or β were generated by injections. A pUAST vector with attB sequence was used to make pUAST-attB-Yki, pUAST-attB-YkiM2, and Yki truncation form flies. Importin α1 mutant fly is a deficiency line from Bloomington Drosophila Stock Center (stock number 25396), which removes the complete importin α1 gene. Importin α1 RNAi is from Bloomington (27523). The following transgenes were used in this study: ex-lacZ, hh-Gal4, GMR-Gal4, UAS-Yki, hpoBF33, YkiB5, which has been described previously (10, 38, 39). All the flies were cultured at 25 °C.
Cell Culture, Transfection, Co-immunoprecipitation, Western Blot, and Luciferase Reporter Assays
S2 cells were maintained in Drosophila Schneider's Medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml of penicillin, and 100 mg/ml of streptomycin. The cells were incubated at 25 °C in a humidified air atmosphere.
Plasmid transfection was carried out using Lipofectamine (Invitrogen), according to the manufacturer's instructions. For all transfection experiments, a ubiquitin-Gal4 construct was co-transfected with the pUAST expression vectors. For immunoprecipitation, after 48 h of expression, cells were lysed in immunoprecipitation buffer (50 mm Tris-HCl, pH 8.0, 100 mm NaCl, 1% Nonidet P-40, 10% glycerol, 1.5 mm EDTA, 10 mm NaF, 1 mm Na3VO4) supplemented with Protease Inhibitor Mixture (Sigma). Cell lysates were pre-cleared by Protein A beads (GE Healthcare) and then incubated with indicated antibodies. Western blot analyses were performed according to standard protocols as previously described. The following antibodies were used in the immunoprecipitation or Western blot analyses: mouse anti-HA (Sigma), mouse anti-Flag (Sigma), mouse anti-Myc (Sigma), and mouse anti-HRP (Santa Cruz Biotechnology) using dilutions of 1:5000 for all antibodies.
For the luciferase reporter assay, the 3xSd2-Luc reporter has been previously described (10). The luciferase assay was performed using the Dual Luciferase Assay System (Promega).
Immunofluorescence Staining
S2 cells and imaginal discs were fixed in 4% formaldehyde (Sigma) and washed three times in PBS supplemented with 0.1% Triton X-100 (PBS-T). The cells and discs were incubated in the primary antibody diluted in PBS-T for 1 h at room temperature, followed by three washes with PBS-T. After the same incubation and wash with a second antibody, cells and discs were mounted in PBS/glycerol medium with DAPI. Primary antibodies used include: mouse anti-Myc (1:500), rabbit anti-Flag (1:200), rabbit anti-HA (1:200), rat anti-Ci (1:250, DSHB), and mouse anti-lacZ (1:500, Invitrogen).
Mass Spectrometry
For each sample, ten 10-cm dishes of S2 cells were collected and lysed in 8 ml of lysis buffer (Tris HCl, pH 8.0, 50 mm, NaCl 100 mm, NaF 10 mm, Na3VO4 1 mm, EDTA 1.5 mm, Nonidet P-40 1%, glycerol 10% supplied with protease inhibitor mixture). Then samples were centrifuged at 14,000 rpm for 20 min at 4 °C, and the supernatant was stored.
GST-tagged YkiN 1–55 amino acid plasmid was made for the protein purification. 0.5 mg of protein was coupled with 500 ml of Affi-Gel10 beads for 4 h at 4 °C. The beads were washed with cold lysis buffer three times and mixed with the cell supernatant at 4 °C for 2 h on a rolling mixer. After 3× washes, the beads were sent directly for MS analysis.
BrdU Incorporation
Drosophila eye discs were dissected and incubated in PBS with 75 μg/ml BrdU for 35 min, then fixed in 5% formaldehyde for 45 min. DNAs were denatured by treating in 3 m HCl for 30 min. Discs were washed three times, and then treated as described for immune staining. Mouse anti-BrdU (Sigma) was used at 1:500 dilution.
Microscopy Image Acquisition and Statistics
Fluorescent microscopy was performed on a Leica LAS SP8 confocal microscope; confocal images were obtained using the Leica AF Lite system. The cells were imaged using a 60× objective, and discs using a 20× objective. All the data were expressed as the mean ± S.E. and were analyzed using Student's t test. Results were considered statistically significant at p < 0.05.
Results
N Terminus of Yki Is Essential for Function and Nuclear Localization
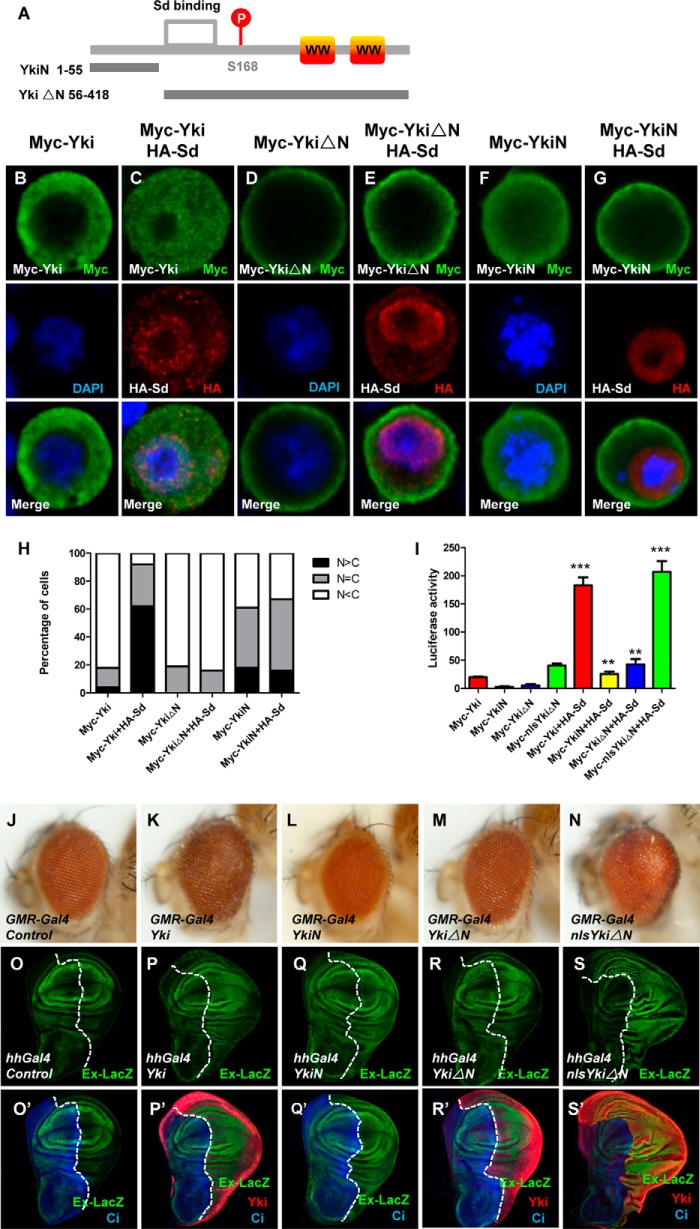
To explore the underlying mechanism of how Yki translocates into the nucleus, we first performed cell-based immunostaining assays. The majority of overexpressed wild-type Yki were maintained in the cytoplasm, while coexpression of Sd caused significant Yki nuclear translocation (Fig. 1, A–C and H). Surprisingly, a truncation of Yki (YkiΔN) that lacks N-terminal 1–55 amino acids mainly distributes in the cytoplasm and near the cell membrane, and even Sd overexpression was not able to drive YkiΔN into the nucleus (Fig. 1, D–E and H). However, the missing part (YkiN) exhibits a dispersive distribution in S2 cells and cannot respond to Sd (Fig. 1, F–G and H).
FIGURE 1.
N terminus of Yki is essential for its nuclear localization and function. A, schematic diagram of Yki and the region corresponding to YkiN/YkiΔN. Yki contains two WW domains. YkiN contains amino acids 1 to 55 and YkiΔN contains amino acids 56 to 418. B–G, S2 cells expressing Myc-Yki (B–C), Myc-YkiN (D–E), Myc-YkiΔN (F–G) with or without HA-Sd were immunostained with anti-Myc (green) or anti-HA (red) antibodies. Nuclei are marked by DAPI (blue). H, cells with different nucleocytoplasmic distributions of Myc-tagged Yki or Yki truncations were counted. A total of 100 cells were counted for each case. The y axis indicates the percentage of cells in each category. I, transcription activities of Yki, YkiN, YkiΔN, and nlsYkiΔN with or without coexpression of Sd in vitro. S2 cells were transfected with the indicated constructs and the reporter genes. 48 h post-transfection, cell lysates were harvested and subjected to a dual luciferase assay. All data are represented as the mean ± S.E. **, p < 0.01. ***, p < 0.001. J–N, side views of wild-type eyes (J) or eyes expressing Yki (K), YkiN (L), YkiΔN (M), or nlsYkiΔN (N) with GMR-Gal4. O–S′, wild-type third-instar larval wing discs (O–O′) or wing discs expressing Yki (P–P′), YkiN (Q–Q′), YkiΔN (R–R′), or nlsYkiΔN (S–S′) under the control of hhGal4 were immunostained to demonstrate the expression of Cubitus (Ci) (blue), Yki (red), Ex-lacZ (green).
To dissect the function of YkiN on Yki cellular localization and activity, a dual luciferase assay was performed using S2 cells (Schneider 2 cells). Co-expression of Yki full-length with Sd synergistically activates the luciferase reporter gene (3xSd2-Luc), while co-expression of YkiN or YkiΔN with Sd failed to induce dramatic activation (Fig. 1I), indicating that the N terminus of Yki is necessary but not sufficient for Yki activity in vitro. To confirm this finding in vivo, transgenic flies expressing Yki variants were generated using the phiC31 integration system, which ensures that the transgenes are expressed at an equal level. Overexpression of Yki full-length by eye-specific driver GMR (glass multiple reporter)-Gal4 caused an overgrowth phonotype (Fig. 1, J–K). As expected, overexpressing YkiN or YkiΔN failed to induce tissue overgrowth (Fig. 1, L–M). In addition, the activity change of the Hippo pathway was examined by detecting the level of ex (expanded)-lacZ, an enhancer trap for ex. Neither YkiN nor YkiΔN that expressed by wing-specific driver hh-Gal4 could induce a significant up-regulation of ex-lacZ as Yki full-length (Fig. 1, O–R′). Conclusively, the N terminus of Yki is necessary but not sufficient for Yki function in Hippo signaling.
What makes YkiN so important in Sd-mediated Yki cytoplasmic-nuclear shuttle? Generally, the nuclear localization of proteins over 50 kDa is mediated by ribosomal synthesis and establishment of the concentration gradient between the cytoplasm and nucleus, which allows proteins that contain an NLS to enter the nucleus (18, 19). We speculated that Yki might contain an NLS at its N terminus. To verify our hypothesis, NLS-YkiΔN, which fuses a NLS from SV40 virus to the 5′-end of YkiΔN protein, was examined by in vitro luciferase assay. NLS-YkiΔN not only exhibits a high activity as Yki full-length but also dramatically activates the reporter upon Sd coexpression (Fig. 1I). Consistently, transgenic flies expressing NLS-YkiΔN have enlarged eyes similar to Yki transgenic flies (Fig. 1N). Moreover, NLS-YkiΔN expression by hh (hedgehog)-Gal4 up-regulated ex-lacZ levels and enhanced P-compartment size substantially (Fig. 1, S–S′). Thus, addition of NLS recused the functional defect of YkiΔN, indicating the N terminus of Yki acts as an NLS to regulate Yki cytoplasmic-nuclear shuttle and hence influences Yki function.
Importin α1 Binds to N Terminus of Yki and Drives Nuclear Translocation of Yki
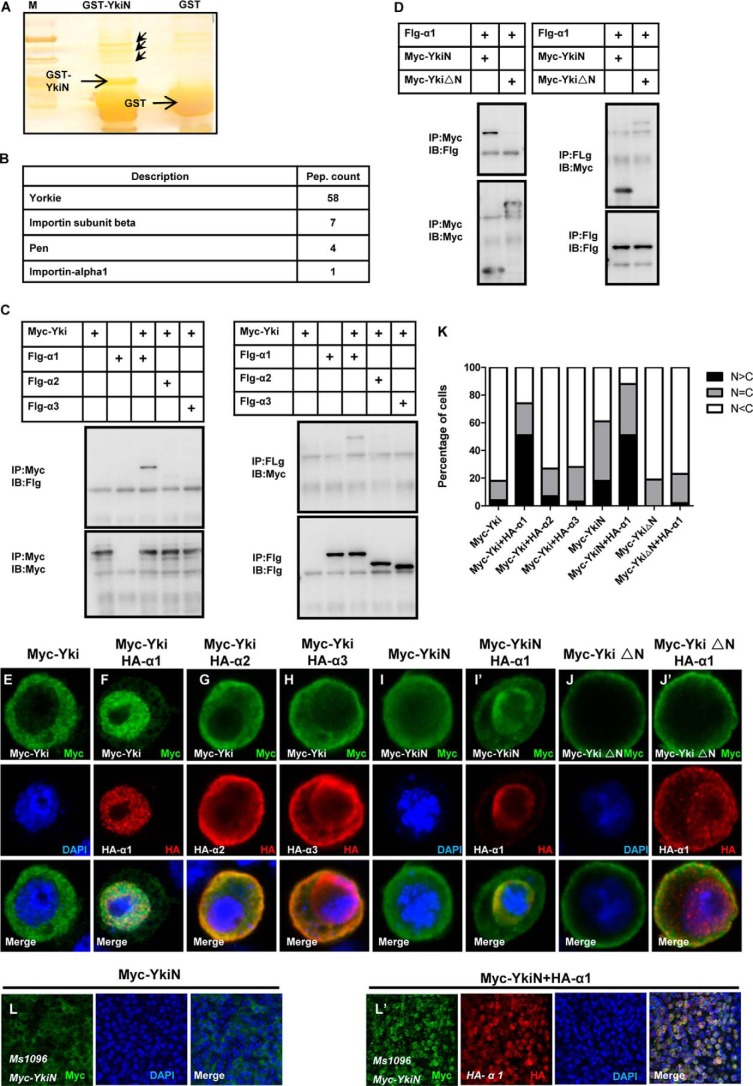
To dissect the regulatory mechanism of Yki cytoplasmic-nuclear translocation, we performed a mass spectrometry (MS) analysis using purified GST-tagged YkiN protein as bait. Two nucleoporin, Importin α1 and pen (Importin α2), have been identified (Fig. 2, A–B). Since YkiN might function as an NLS (Fig. 1), we deduced that the Yki cytoplasmic-nuclear translocation may be through the nucleoporin complex. To test this hypothesis, we constructed HA-tagged α1 (α1), Importin α2 (α2), and Importin α3 (α3) for co-immunoprecipitation assay. Interestingly, only α1 directly interacted with Yki in two directions (Fig. 2C). To narrow down the binding region of Yki-α1, we detected the association between YkiN/YkiΔN and α1. YkiN has a strong association with α1 in two directions, whereas no distinct interaction of YkiΔN-α1 was observed (Fig. 2D). It is feasible to consider that Importin α1 drives Yki nuclear translocation. As shown in Fig. 2, E–H and K, upon α1 coexpression, the majority of Yki translocates into the nucleus. Of note, α2 or α3 coexpression did not cause a similar change, consistent with the immunoprecipitation results in Fig. 2C. In addition, α1 only changes the localization of YkiN but not YkiΔN (Fig. 2, I–J′ and K). Importantly, a clear and profound nuclear import of YkiN in vivo can be detected (Fig. 2, L–L′). However, no dramatic nuclear localization of Yki full-length upon α1 expression was observed in vivo (data not show). We speculated that Yki full-length is functional in vivo, and its nuclear translocation is tightly regulated by multiple factors and mechanisms. In toto, we conclude that Importin α1 drives Yki nuclear localization via binding to its N terminus.
FIGURE 2.
Importin α1 binds to the N terminus of Yki and mediates the nuclear localization of Yki. A, silver staining of the sample for MS. GST and GST-YkiN protein were purified and coupled with GST beads, then pull-down using the S2 cell lysate. The specific bands are shown with red arrowhead. B, results for MS. MS analysis have identified Importin α1, pen (Importin α2), Importin β. C, Importin α1 interacts with Yki in vitro. S2 cells were transfected with the indicated constructs. The cell lysates were immunoprecipitated and subjected to Western blot analysis. D, Importin α1 only interacts with YkiN. S2 cells expressing Myc-YkiN, Myc-YkiΔN, and Flg-Importin α1 were immunoprecipitated and probed with the indicated antibodies. E–H, S2 cells expressing Myc-Yki (E) or Myc-Yki with HA-Importin α1 (F), HA-Importin α2 (G), HA-Importin α3 (H) were immunostained with anti-Myc (green) or anti-HA (red) antibodies. I–J′, S2 cells expressing Myc-YkiN (I–I′), Myc-YkiΔN (J–J′) with or without HA-Importin α1 were immunostained with anti-Myc (green) or anti-HA (red) antibodies. Nuclei are marked by DAPI (blue). K, cells with different nucleocytoplasmic distributions of Myc-tagged variants were counted. A total of 100 cells were counted for each case. The y axis indicates the percentage of cells in each category. L–L′, Drosophila imaginal wing discs expressing Myc-YkiN (L) or Myc-YkiN plus HA-Importin α1 (L′) by MS1096 were dissected and stained with anti-Yki (green) or anti-HA (red) antibodies.
Importin α1 Is Indispensable for Hippo Signaling in Vivo
To verify whether Importin α1 is a bona fide Yki regulator in vivo, we developed an importin α1-null fly line. Overexpression of UAS-Yki from the posterior to the morphogenetic furrow (MF) using the GMR-Gal4 driver (GMR-Yki) resulted in enlarged eyes, while expression of Yki in importin α1 hypomorphic mutant flies (α1±) led to smaller and smooth eyes (Fig. 3, A–C′). BrdU and Diap1, markers of proliferation and anti-apoptosis, were increased dramatically when Yki was overexpressed while decreased in α1± background (Fig. 3, A″–C‴). Collectively, the inactivation of Importin α1 suppressed Yki function. To strengthen this conclusion, the expression level of Diap1, which reflects Yki activity, was examined in Importin α1 mosaic clones. In α1−/− MARCM (Mosaic analysis with a repressible cell marker) clones, Yki overexpression-induced up-regulation of Diap1 is significantly blocked (Fig. 3, D–F‴). Importantly, the sizes of α1−/−-null clones were small, even when Yki was overexpressed, indicating a proliferative disadvantage of α1−/−-null clones. However, considering the large number of Importin α1 targets, there might be other collaborating transcription factors, such as E2F or Mad, which also shuttles, to join in this processes. Furthermore, we analyzed the genetic epistasis between hpo (hippo) and Importin α1. In hpo mutant clones induced by the MARCM system, the ablation of hpo resulted in tumor-like overgrowth and elevated the protein levels of Ex, while these changes can be markedly suppressed by Importin α1RNAi (Fig. 3, G–I‴). These observations suggest that Importin α1 acts downstream of hpo to influence Hippo signaling.
FIGURE 3.
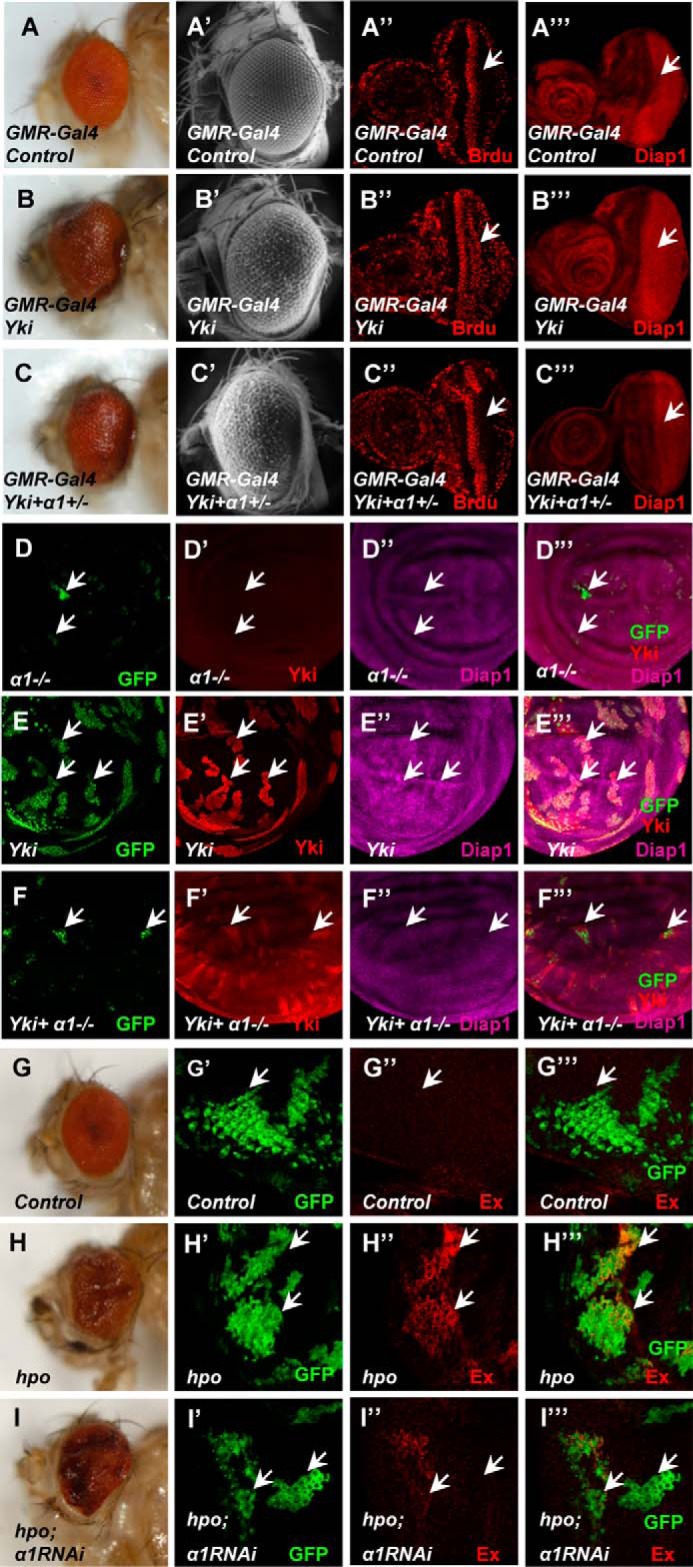
In vivo, the function of Hippo signaling is dependent on Importin α1. A–C‴, one copy of Importin α1 mutant partially blocks Yki function. Side view (A–C), electron microscope photos (A′–C′), BrdU incorporation (A″–C″), or Diap1 expression (A‴–C‴) of wild-type eyes (A–A‴) or eyes expressing Yki (B–B‴) or Yki plus Importin α1± (C–C‴) by GMR-Gal4. (D–F‴) Importin α1-null clones block the function of Yki. Larval wing discs containing the Importin α1 mutant (D–D‴), Yki overexpression (E–E‴), Importin α1 mutant combined with Yki (F–F‴) were dissected and examined to determine the expression of Yki (D′–F′) and Diap1 (D″–F″). Importin α1 mutant clones were marked by GFP signal. G–I‴) Importin α1 RNAi can partially block the function of the hpo mutant. Side views of adult eyes contain Importin α1 RNAi only (G), hpo mutant only (H), hpo mutant clones plus Importin α1 RNAi (I). Drosophila imaginal eye discs expressing Importin α1 RNAi (G′-G‴), hpo mutant clones (H′-H‴), or hpo mutant clones plus Importin α1RNAi (I′-I‴) using the MARCM system were dissected and stained with anti-GFP (green) or anti-Ex (red) antibodies.
Yki R15 Is Critical for Yki Association with Importin α1 and Its Cellular Localization
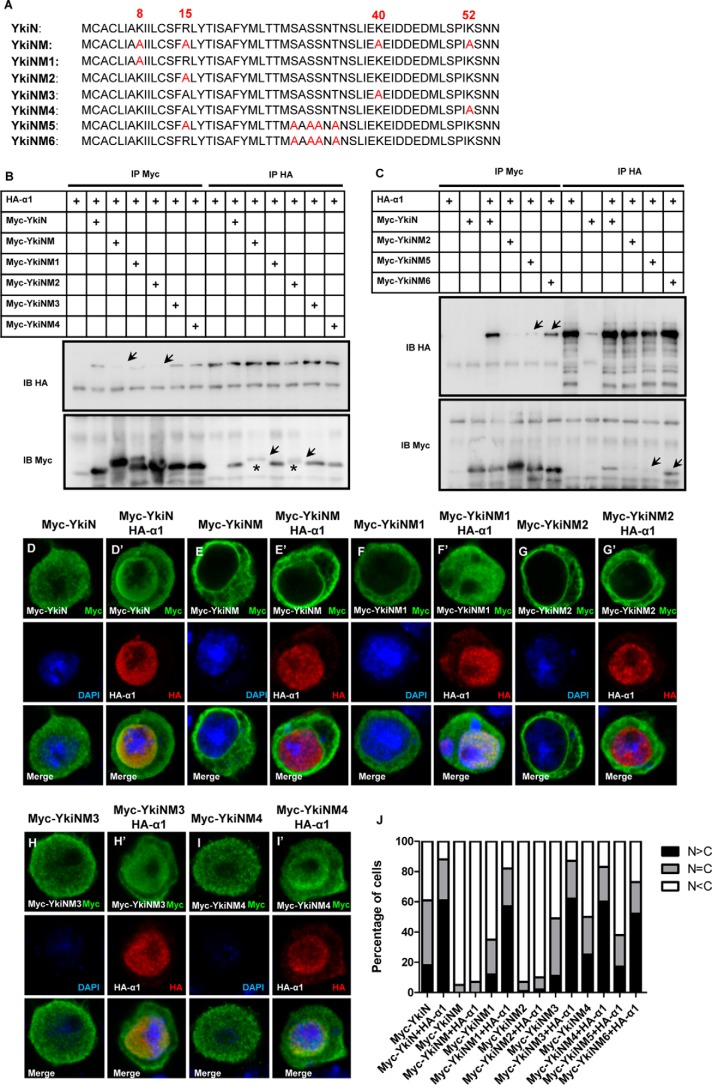
We have shown the indispensable roles of the Yki N terminus and Importin α1 in nuclear translocation of Yki. To dissect how Importin α1 regulates Yki function, we first mapped the N terminus of Yki. It has been reported that the classic NLS motif always contains several lysine and arginine repeats, but some untypical NLS may only contain one or two lysines or arginines (20). No classical NLS sequence was found in YkiN, but there are three lysines and one arginine residue (Lys-8, Arg-15, Lys-40, and Lys-52) that could be important for nuclear import (Fig. 4A). YkiN variants that contain point mutation of these sites together or individually were generated and examined (Fig. 4A). As expected, YkiNM, which contains four mutations together, failed to interact with Importin α1 (Fig. 4B), and localized mainly in the cytoplasm with an accumulation near the cell and nuclear membranes (Fig. 4, E–E′). Strikingly, YkiNM2, which bears a single point mutation of R15A, blocks Yki-Importin α1 association in two directions, whereas the other three single mutations had no effect on Yki-Importin α1 interaction (Fig. 4, A–B). Also, YkiNM2, but not the other three, exhibited a similar localization to YkiNM, which failed to respond to Importin α1 (Fig. 4, F–J).
FIGURE 4.
Mapping the important sites on the Yki N terminus. A, amino acid sequence of YkiN/YkiNM/YkiNM1/YkiNM2/YkiNM3/YkiNM4/YkiNM5/YkiNM6. B, R15A point mutation of YkiN (YkiNM2) blocks its association with Importin α1. S2 cells were transfected with the indicated plasmids and were immunoprecipitated, followed by Western blot analysis. C, Myc-YkiN and Myc-YkiNM6 can interact with Importin α1, while Myc-YkiNM2 or Myc-YkiNM5 cannot. S2 cells were transfected with the indicated constructs. The cell lysates were immunoprecipitated and probed by anti-Myc and anti-HA antibodies. D–I′, YkiNM2 shows suppressed nuclear entrance induced by Importin α1. S2 cells were transfected with the YkiN variants and Importin α1 and then stained with anti-Myc (green) and anti-HA (red) antibodies. J, cells in D–I′ were categorized based on the anti-Myc immunostaining pattern. 100 cells were counted in each case. S2 cells expressing the indicated proteins were immunostained with the anti-Myc (green) and anti-HA (red) antibodies.
However, we noticed a mobility shift of Yki mutants bearing the R15A mutation (YkiNM2) (Fig. 4B). We speculated that the change of Arg-15 might induce changes in Ser or Thr phosphorylation status in YkiN. We classified thirteen Ser and Thr on YkiN into groups, generated mutants accordingly, and identified the point mutation combination in YkiNM5 that blocked the shift band caused by R15A mutation (Fig. 4, A and C). Subsequently, we tested whether the phosphorylation status of these sites influences YkiN localization using the YkiNM6 mutant, which contains the same Ser/Thr mutations as YkiNM5 but lacks the Arg-15 mutation (Fig. 4, A and C). Unlike YkiNM5, YkiNM6 still interacts with Importin α1 and is able to translocate into the nucleus like wild-type YkiN (Fig. 4J). Collectively, the mobility shift of Yki mutants bearing R15A mutation does not affect its nuclear translocation.
To test the function of Yki Arg-15, the differences among Yki and YkiM2 (full-length Yki bearing the R15A mutation) in the interaction with Importin α1 and cellular localization were examined. Consistently, the mutation of Arg-15 in full-length Yki also disrupts its association with Importin α1 and showed a similar cellular localization as YkiNM2 (Fig. 5, A and B–F). We then checked the functional difference in vivo using the transgenic fly expressing equal levels of Yki and YkiM2 in the MARCM system. Because Yki was an important factor in growth control, yki-null clones have very small size and obviously decreased Diap1 level (Fig. 5, G–H‴). Upon overexpression of Yki, the clone size and Diap1 level were dramatically increased (Fig. 5, I–I‴), suggested a high activity of overexpressed wild-type Yki. However, YkiM2 showed no influence on the clone size and Diap1 level for yki-null clones, (Fig. 5, J–J‴), indicating YkiM2 exhibits a functional defect and hence was not able to rescue yki mutant clones. This compelling evidence strongly supports our finding that amino acid residue Arg-15 of Yki is important for the function of Yki in vivo. In other words, the localization of Yki and/or its binding ability with Importin α1 is critical for the function of Yki.
FIGURE 5.
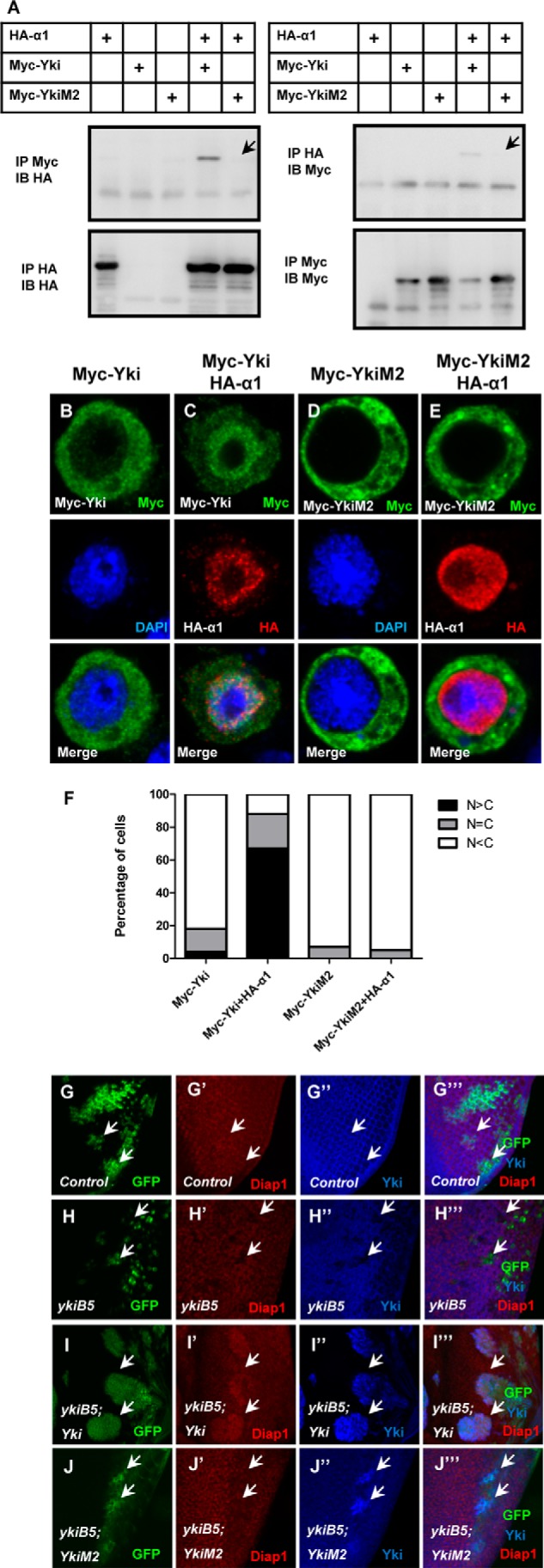
Test the function of Arg-15 in vitro and in vitro. A, Yki full-length point mutation (YkiM2) has no interaction with Importin α1. Co-immunoprecipitation between Myc-Yki/YkiM2 and HA-Importin α1 is performed. B–E, YkiM2 stays in the cytoplasm when Importin α1 was co-expressed. Immunostaining of S2 cells expressing Myc-Yki (B–C) or Myc-YkiM2 (D–E) (green) with or without HA-Importin α1 (red). F, cells in B–E were categorized based on the anti-Myc immunostaining pattern. 100 cells were counted in each case. G–J‴, YkiM2 have a functional defect in vivo. Drosophila imaginal eye discs expressing wide type clones (G–G‴), yki mutant clones (H–H‴), yki mutant clones plus overexpressed Yki (I–I‴) or yki mutant clones plus overexpressed YkiM2 (J–J‴) using the MARCM system were dissected and stained with anti-Diap1 (red) or anti-Yki (blue) antibodies.
Hippo Signaling Regulates Yki Localization via Impairing the Binding between Yki and Importin α1
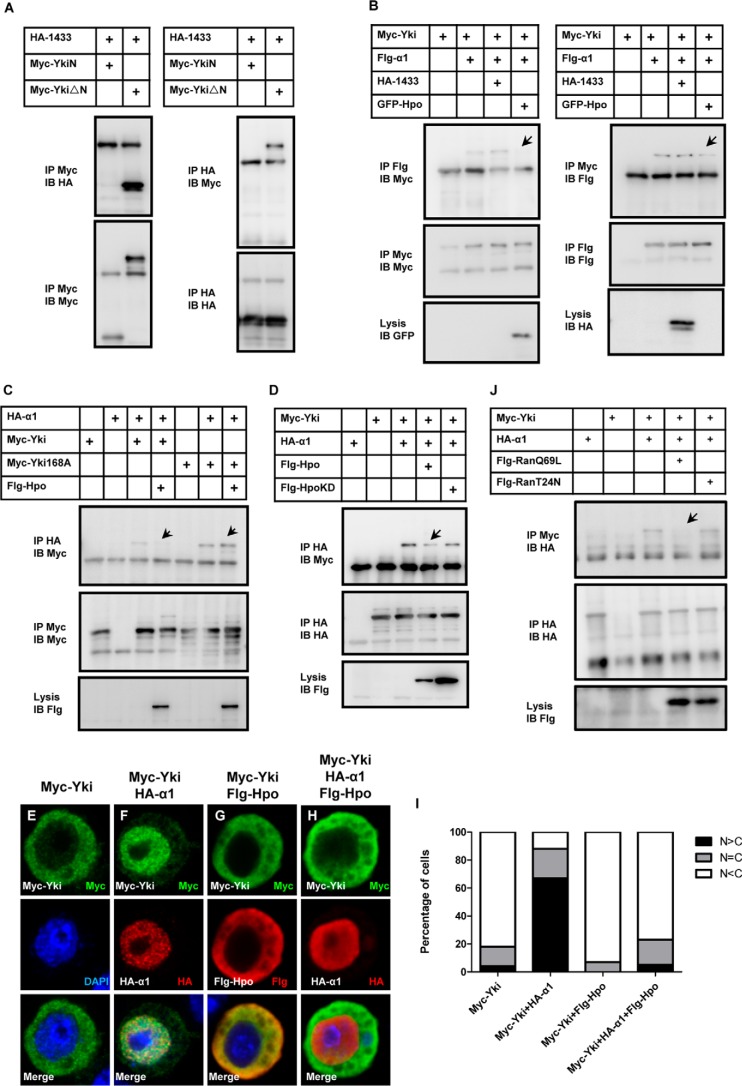
The Hippo pathway regulates the activity of Yki by tight control of its cytoplasmic-nuclear shuttle by inducing physical interaction with 14-3-3 proteins (40). Here we raise the question of whether Importin α1 associates with 14-3-3, or whether they affect each other? As shown in Fig. 6A, 14-3-3 interacts with YkiΔN, but not YkiN. In addition, the interaction between Yki and Importin α1 was not affected by 14-3-3 coexpression (Fig. 6B). These observations suggest that Importin α1 and 14-3-3 regulate Yki cellular localization via different mechanisms by binding different regions.
FIGURE 6.
Hippo signaling regulates Yki localization by impairing the binding between Yki and Importin α1. A, 14-3-3 cannot interact with YkiN, but can interact with YkiΔN. B, Hpo but not 14-3-3 affects the binding between Yki and Importin α1. S2 cells expressing the indicated constructs were immunoprecipitated and probed with the indicated antibodies. C, cellular localization of Myc-Yki but not that of Myc-Yki168A is influenced by Hpo. Co-immunoprecipitation experiment was performed, followed by Western blot analysis. D, function of Hpo depends on its kinase activity. S2 cells were transfected with the indicated constructs. The cell lysates were immunoprecipitated, followed by Western blot. E–H) Hpo inhibits Importin α1 induced Yki localization. S2 cells were immunostained with the indicated antibodies. I, cells in E–H were categorized based on the anti-Myc immunostaining pattern. 100 cells were counted in each case. J, interaction between Yki and Importin α1 was Ran-GTP sensitive. S2 cells were transfected with the indicated constructs. The cell lysates were immunoprecipitated, followed by Western blot analysis.
The role of Hpo kinase in Importin α1-mediated Yki localization regulation was also explored. As shown in Fig. 6B, the binding between Yki and Importin α1 was attenuated when Hpo was coexpressed. YkiS168A is reported to escape Hippo signaling-mediated regulation and has a higher level of nuclear localization (41, 42). Indeed, YkiS168A exhibits a stronger association with Importin α1, and coexpression of Hpo failed to affect the binding (Fig. 6C). Moreover, in the competitive binding assay, Hpo kinase activity is required for regulating Yki-Importin α1 association (Fig. 6D). As shown in Fig. 6, E–I, Hpo causes Yki retardation in the cytoplasm even when Importin α1 is coexpressed. These findings indicated a dominant role of Hpo in Importin α1-dependent Yki nuclear translocalization. Moreover, we tested the role of Ran in Yki localization regulation. As shown in Fig. 6J, the interaction between Yki and Importin α1 decreased upon RanQ69L (Ran-GTP) addition, while the interaction was not affected by addition of RanT24N (Ran-GDP), suggesting that the binding between Yki and Importin α1 is Ran-GTP sensitive.
Discussion
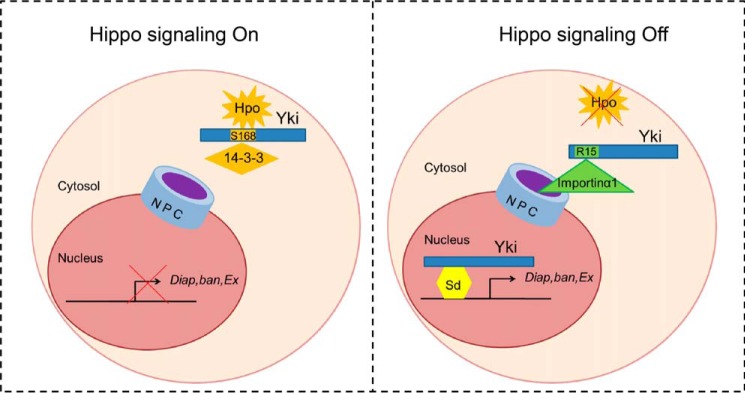
It has been reported that the Hippo pathway plays essential roles in organ size control, stem cell maintenance, tissue homeostasis, and repair (2, 43–45). The nuclear import of Yki, or its mammalian homolog Yap, is a critical step for Hippo signaling transduction. However, little is known about the mechanism of this process. Based on the MS screen results, we demonstrated that Importin α1 might be involved in Yki nuclear translocation through interaction with the N terminus of Yki. The nuclear import of most proteins by Importin α1 requires a classical NLS (46, 47). We proposed that although Yki did not contain such a motif, the N-terminal 1–55 amino acids of Yki, especially Arg-15, act as an NLS to interact with Importin α1 and mediate the nuclear translocation of Yki. Furthermore, by testing the regulation from the upstream kinase Hpo, the close relationship between Importin α1 and Hippo pathway was revealed (Fig. 7).
FIGURE 7.
Model of how Importin α1 mediates Yki nuclear import. Schematic diagram of the regulation of Yki nuclear import. When Hippo signaling is on, the upstream kinase can phosphorylate Yki at its Ser-168, making Yki stay in the cytoplasm through binding with 14-3-3 (left); while when Hippo signaling is off, Importin α1 can bind Yki through its Arg-15, then bring Yki into the nucleus to induce target gene expression (right).
The Hippo signaling pathway suppresses Yki nuclear localization by mediating Yki phosphorylation, which promotes the binding between Yki and 14-3-3 proteins to retain Yki in the cytoplasm (40, 41). Here we raise the question of whether importin α1 associates with 14-3-3 or whether the two proteins affect each other. First, we showed that the interaction of Yki and 14-3-3 is independent of the Yki N-terminal region. Subsequently, we demonstrated that 14-3-3 did not affect the binding between Yki and Importin α1. We propose that Importin α1 and 14-3-3 regulate Yki nuclear translocalization through different mechanisms.
According to the immunostaining data in S2 cells (Fig. 2), Importin α1 can promote the nuclear localization of Yki full-length and YkiN dramatically. However, when performing the same experiment in Drosophila imaginal discs, we only observed a similar trend of nuclear localization of YkiN, but not that of Yki full-length. The explanation for this observation would be that the process of Yki nuclear-cytoplasmic shuttle was tightly regulated by multiple mechanisms.
The data in Fig. 1, D–E show that deleting the N-terminal region of Yki not only prevents Yki nuclear translocation but also enhances Yki cortical localization near the outer cell membrane. One possibility might be that YkiN can bind factors other than Importin α1 to prevent membrane association. In MS analysis, we discovered multiple candidates of possible factors. Further experiments are needed to explore the underlying mechanism regarding this matter.
An evolutionally conserved function of Yki in growth control has been reported. To determine whether the function of Importin α1 is conserved, the human homolog of Importin α1, KPNA6 was cloned. However, when co-expressed Yap and KPNA6 in 293T cells or MCF10A cells, Yap did not show higher levels of nuclear localization. In addition, no physical interaction was detected in 293T cells (data not shown). It has been reported that more than twenty Importins exist in the human. Therefore, Yap nuclear translocation may also be mediated by other Importin members or by additional mechanisms (27, 48).
Since the nuclear localization of Yap is a remarkable marker in many kinds of human cancers, it will be meaningful to investigate the Yap nuclear import regulation. Further studies on YAP translocalization might help to solve this puzzle.
Author Contributions
S. W., Y. L., M.-X. Y. and L. Z. conceived and designed the experiments; S. M., Y. L., M.-X. Y., C. W. performed the experiments; S. M., Y. L., M.-X. Y., C. W., L. Z. analyzed the data; W. W., J. H., WQ. W., L. H., L. G. contributed regents/materials; S. M., M.-X. Y., and L. Z. wrote the paper.
Acknowledgments
We thank Shi-an Wu and the Bloomington for related fly stocks.
This work was supported by grants from the National Basic Research Program of China (973 Program 2012CB945001), the “Strategic Priority Research Program” of the Chinese Academy of Sciences (XDA01010406), the National Natural Science Foundation of China (31171394, 31371462), supported by the State Key Program of National Natural Science of China (31530043), the “Cross and Cooperation in Science and Technology Innovation Team” project of the Chinese Academy of Sciences, and the CAS/SAFEA International Partnership Program for Creative Research Teams. The authors declare that they have no conflicts of interest with the contents of this article.
- Yki
- Yorkie
- YAP
- yes-associated protein
- Sd
- Scalloped
- NPC
- nuclear pore complex
- NLS
- nuclear localization signal.
References
- 1.Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S. A., Gayyed M. F., Anders R. A., Maitra A., and Pan D. (2007) Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin M., and Zhang L. (2011) Hippo signaling: a hub of growth control, tumor suppression and pluripotency maintenance. J. Genet. Genom. 38, 471–481 [DOI] [PubMed] [Google Scholar]
- 3.Yin M. X., and Zhang L. (2015) Hippo signaling in epithelial stem cells. Acta Biochim. Biophys. Sin 47, 39–45 [DOI] [PubMed] [Google Scholar]
- 4.Harvey K. F., Pfleger C. M., and Hariharan I. K. (2003) The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457–467 [DOI] [PubMed] [Google Scholar]
- 5.Wu S., Huang J., Dong J., and Pan D. (2003) hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445–456 [DOI] [PubMed] [Google Scholar]
- 6.Pantalacci S., Tapon N., and Léopold P. (2003) The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5, 921–927 [DOI] [PubMed] [Google Scholar]
- 7.Udan R. S., Kango-Singh M., Nolo R., Tao C., and Halder G. (2003) Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5, 914–920 [DOI] [PubMed] [Google Scholar]
- 8.Xu T., Wang W., Zhang S., Stewart R. A., and Yu W. (1995) Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053–1063 [DOI] [PubMed] [Google Scholar]
- 9.Justice R. W., Zilian O., Woods D. F., Noll M., and Bryant P. J. (1995) The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534–546 [DOI] [PubMed] [Google Scholar]
- 10.Zhang L., Ren F., Zhang Q., Chen Y., Wang B., and Jiang J. (2008) The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell 14, 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goulev Y., Fauny J. D., Gonzalez-Marti B., Flagiello D., Silber J., and Zider A. (2008) SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr. Biol. 18, 435–441 [DOI] [PubMed] [Google Scholar]
- 12.Wu S., Liu Y., Zheng Y., Dong J., and Pan D. (2008) The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell 14, 388–398 [DOI] [PubMed] [Google Scholar]
- 13.Nolo R., Morrison C. M., Tao C., Zhang X., and Halder G. (2006) The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr. Biol. 16, 1895–1904 [DOI] [PubMed] [Google Scholar]
- 14.Thompson B. J., and Cohen S. M. (2006) The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell 126, 767–774 [DOI] [PubMed] [Google Scholar]
- 15.Peng H. W., Slattery M., and Mann R. S. (2009) Transcription factor choice in the Hippo signaling pathway: homothorax and yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev. 23, 2307–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran E. J., and Wente S. R. (2006) Dynamic nuclear pore complexes: life on the edge. Cell 125, 1041–1053 [DOI] [PubMed] [Google Scholar]
- 17.Macara I. G. (2001) Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65, 570–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Görlich D., Panté N., Kutay U., Aebi U., and Bischoff F. R. (1996) Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 15, 5584–5594 [PMC free article] [PubMed] [Google Scholar]
- 19.Jäkel S., and Görlich D. (1998) Importin beta, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 17, 4491–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosugi S., Hasebe M., Matsumura N., Takashima H., Miyamoto-Sato E., Tomita M., and Yanagawa H. (2009) Six classes of nuclear localization signals specific to different binding grooves of importin α. J. Biol. Chem. 284, 478–485 [DOI] [PubMed] [Google Scholar]
- 21.Lange A., Mills R. E., Lange C. J., Stewart M., Devine S. E., and Corbett A. H. (2007) Classical nuclear localization signals: definition, function, and interaction with importin α. J. Biol. Chem. 282, 5101–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins J., Dilworth S. M., Laskey R. A., and Dingwall C. (1991) Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 64, 615–623 [DOI] [PubMed] [Google Scholar]
- 23.Dingwall C., and Laskey R. A. (1991) Nuclear targeting sequences–a consensus? Trends Biochem. Sci. 16, 478–481 [DOI] [PubMed] [Google Scholar]
- 24.Fontes M. R., Teh T., Toth G., John A., Pavo I., Jans D. A., and Kobe B. (2003) Role of flanking sequences and phosphorylation in the recognition of the simian-virus-40 large T-antigen nuclear localization sequences by importin-α. Biochem. J. 375, 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodel M. R., Corbett A. H., and Hodel A. E. (2001) Dissection of a nuclear localization signal. J. Biol. Chem. 276, 1317–1325 [DOI] [PubMed] [Google Scholar]
- 26.Makkerh J. P., Dingwall C., and Laskey R. A. (1996) Comparative mutagenesis of nuclear localization signals reveals the importance of neutral and acidic amino acids. Curr. Biol. 6, 1025–1027 [DOI] [PubMed] [Google Scholar]
- 27.Wagstaff K. M., and Jans D. A. (2009) Importins and beyond: non-conventional nuclear transport mechanisms. Traffic 10, 1188–1198 [DOI] [PubMed] [Google Scholar]
- 28.Stewart M. (2007) Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell Biol. 8, 195–208 [DOI] [PubMed] [Google Scholar]
- 29.Conti E., Uy M., Leighton L., Blobel G., and Kuriyan J. (1998) Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin α. Cell 94, 193–204 [DOI] [PubMed] [Google Scholar]
- 30.Conti E., and Kuriyan J. (2000) Crystallographic analysis of the specific yet versatile recognition of distinct nuclear localization signals by karyopherin α. Structure 8, 329–338 [DOI] [PubMed] [Google Scholar]
- 31.Fontes M. R., Teh T., Jans D., Brinkworth R. I., and Kobe B. (2003) Structural basis for the specificity of bipartite nuclear localization sequence binding by importin-α. J. Biol. Chem. 278, 27981–27987 [DOI] [PubMed] [Google Scholar]
- 32.Marfori M., Lonhienne T. G., Forwood J. K., and Kobe B. (2012) Structural basis of high-affinity nuclear localization signal interactions with importin-α. Traffic 13, 532–548 [DOI] [PubMed] [Google Scholar]
- 33.Török I., Strand D., Schmitt R., Tick G., Török T., Kiss I., and Mechler B. M. (1995) The overgrown hematopoietic organs-31 tumor suppressor gene of Drosophila encodes an Importin-like protein accumulating in the nucleus at the onset of mitosis. J. Cell Biol. 129, 1473–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Küssel P., and Frasch M. (1995) Pendulin, a Drosophila protein with cell cycle-dependent nuclear localization, is required for normal cell proliferation. J. Cell Biol. 129, 1491–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Máthé E., Bates H., Huikeshoven H., Deák P., Glover D. M., and Cotterill S. (2000) Importin-α3 is required at multiple stages of Drosophila development and has a role in the completion of oogenesis. Dev. Biol. 223, 307–322 [DOI] [PubMed] [Google Scholar]
- 36.Mason D. A., Fleming R. J., and Goldfarb D. S. (2002) Drosophila melanogaster importin α1 and α3 can replace importin α2 during spermatogenesis but not oogenesis. Genetics 161, 157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorjánácz M., Adám G., Török I., Mechler B. M., Szlanka T., and Kiss I. (2002) Importin-α2 is critically required for the assembly of ring canals during Drosophila oogenesis. Dev. Biol. 251, 271–282 [DOI] [PubMed] [Google Scholar]
- 38.Huang H. L., Wang S., Yin M. X., Dong L., Wang C., Wu W., Lu Y., Feng M., Dai C., Guo X., Li L., Zhao B., Zhou Z., Ji H., Jiang J., Zhao Y., Liu X. Y., and Zhang L. (2013) Par-1 regulates tissue growth by influencing hippo phosphorylation status and hippo-salvador association. PLos Biol. 11, e1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang J., Wu S., Barrera J., Matthews K., and Pan D. (2005) The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122, 421–434 [DOI] [PubMed] [Google Scholar]
- 40.Ren F., Zhang L., and Jiang J. (2010) Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev. Biol. 337, 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh H., and Irvine K. D. (2008) In vivo regulation of Yorkie phosphorylation and localization. Development 135, 1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh H., and Irvine K. D. (2009) In vivo analysis of Yorkie phosphorylation sites. Oncogene 28, 1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao B., Li L., Lei Q., and Guan K. L. (2010) The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 24, 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao B., Tumaneng K., and Guan K. L. (2011) The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13, 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan D. (2010) The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorokin A. V., Kim E. R., and Ovchinnikov L. P. (2007) Nucleocytoplasmic transport of proteins. Biochemistry 72, 1439–1457 [DOI] [PubMed] [Google Scholar]
- 47.Poon I. K., and Jans D. A. (2005) Regulation of nuclear transport: central role in development and transformation? Traffic 6, 173–186 [DOI] [PubMed] [Google Scholar]
- 48.Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., and Adam S. A. (2004) Importin α: a multipurpose nuclear-transport receptor. Trends Cell Biol. 14, 505–514 [DOI] [PubMed] [Google Scholar]