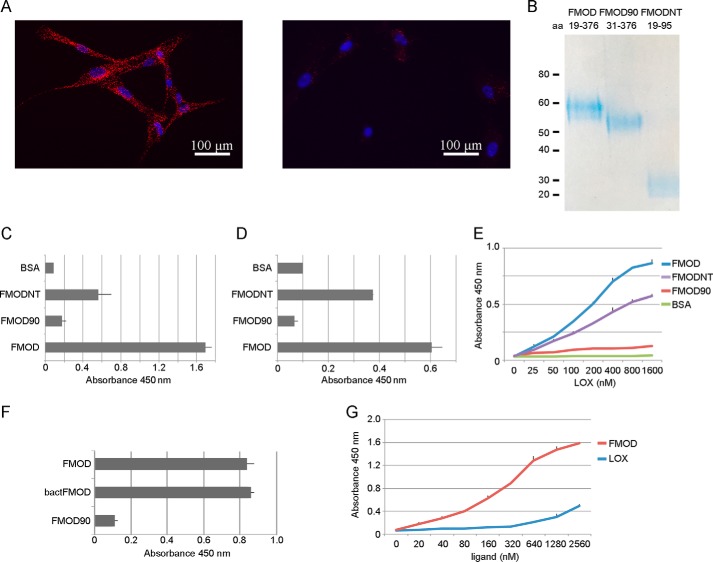
FIGURE 2.
Identification and characterization of the FMOD-LOX interaction. A, proximity ligation assay on HFL-1 cells expressing FMOD (left panel) or non-collagen-binding His-tagged FMOD fragment as a negative control (right panel). Anti-His tag and anti-LOX antibodies were used to detect the interaction (red fluorescence). Nuclear staining with DAPI is shown in blue. B, gel image of FMOD variants used in the solid-phase assays in C–F. The proteins were expressed in 293T cells and purified on Ni-NTA-Sepharose. The amino acid (aa) range of each construct is listed. C, solid-phase assays showing interactions between FMOD variants and LOX. LOX was coated on a 96-well plate that was blocked with BSA and incubated with 170 nm FMOD variants, whose binding was detected using anti-His tag antibody. D, as in C, but here FMOD variants were coated on the plate followed by incubation with 150 nm LOX and detection with anti-LOX antibody. The experiments in C and D were repeated twice, giving similar results. E, as in D, but here serial dilutions of LOX were used to determine the apparent KD. Similar results were obtained in three experiments. F, solid-phase assay to assess binding of LOX to coated FMOD (expressed in 293 cells), bactFMOD (expressed in E. coli), and FMOD90. The assay was performed as in D. G, solid-phase assay to compare binding of serially diluted FMOD and LOX to coated collagen in a 96-well plate. Binding was detected using anti-His tag or anti-LOX antibodies. All error bars represent mean ± S.D. (n = 3 technical replicates). The experiments in F and G gave similar results on three occasions.