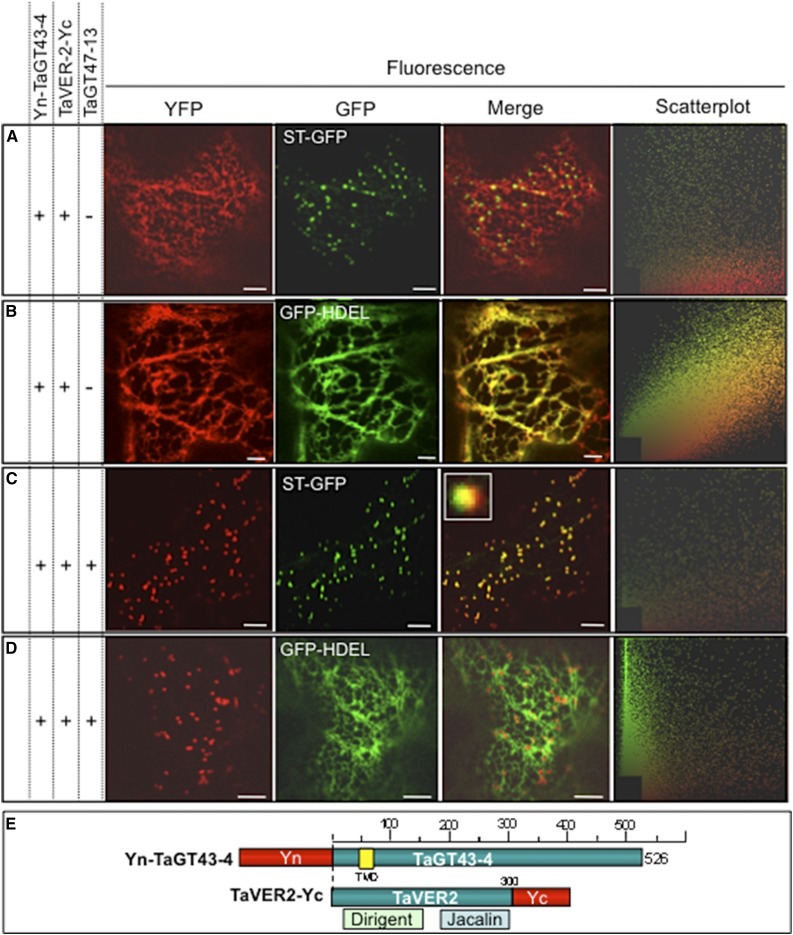
Figure 6.
TaGT43-4, TaVER2, and TaGT47-13 assemble in the ER before export to the Golgi. Protein-protein interactions were investigated using BiFC (split-YFP). The data show that TaGT43-4 interacts with TaVER2 in the ER to form a complex that is retained in the ER (A and B) until interaction with untagged TaGT47-13, which results in the export of the complex from the ER to a Golgi compartment that has partial overlap with trans-Golgi (C and D). ST-GFP and GFP-HDEL, and trans-Golgi and ER markers were used to show the colocalization of the assembled YFP. GFP and YFP fluorescence are shown in green and red, respectively, and their colocalization (merge) appears in yellow. Two-dimensional scatterplots on the right display the degree of overlap between the red and green in the images. The inset in merge picture in C shows the limited overlap between YFP and ST-GFP. Bars = 10 μm. Schematic presentations of YFP-tagged constructs used in this study are shown in E. TaVER2 is a soluble protein containing two domains: a dirigent domain at the NH2-terminal end and a lectin domain (Jacalin) at the COOH-terminal end.