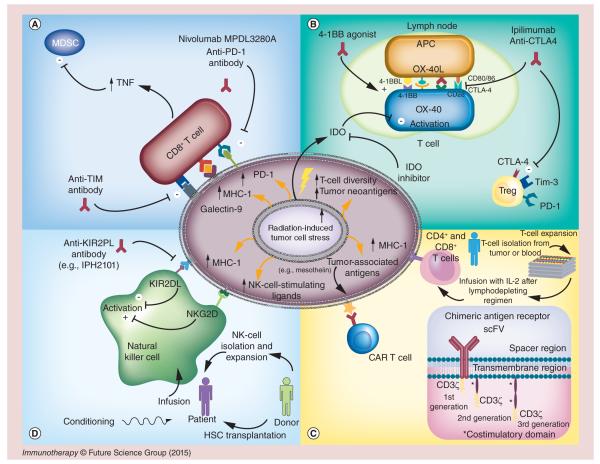
Figure 1. Radiation and immunotherapy effects on the tumor microenvironment.
(A, top left) T-cell and tumor interaction. PDL1 and galectin-9 expressed on the tumor-cell surface bind to PD1 and Tim-3 on CD8+ T cells to blunt the immunologic response of those T cells. Tim-3 also stimulates T-regulatory (Treg) cells. PDL1 expression can be increased after irradiation. Blocking the PDL1 axis reinvigorates CD8+ T-cell activation. Concurrent use of PDL1 blockade with radiation results in increased TNF-α, which contributes to killing of myeloid-derived suppressor cells (MDSCs), further improving T-cell function. Antibodies blocking PD1 (nivolumab and pembrolizumab) and Tim-3 reduce the population of dysfunctional CD8+ T cells, resulting in improved tumor control. (B, top right) T-cell activation at a lymph node. Antigen-presenting cells (APCs) travel to the lymph node from the tumor and activate T cells by presenting tumor antigens to the T cells. CTLA4 or CD28 can bind to CD80 (or CD86) on the T cell, serving as a regulator to balance T-cell activity. Ipilimumab and tremelimumab work at the lymph node to block CTLA4 binding with CD80 (CD86), which causes Treg cell death and CD8+ T-cell expansion. Radiation promotes T-cell receptor diversity when added to anti-CTLA4 therapy. Meanwhile, OX40 and 4-1BB agonists both contribute to T-cell stimulation in the lymph node, and IDO, secreted by tumor cells, depletes T cells of tryptophan to metabolically induce T-cell death. (C, bottom right) T-cell therapy at the tumor site. (1) Tumor-associated antigens, upregulated after irradiation, are recognized by the T-cell receptor of infused T cells. Adoptive T-cell therapy is initiated by extracting T cells from blood or tumor samples and then isolating and expanding them in tissue culture with IL-2. When enough cells have accumulated, they are tested for viability, tumor specificity and contamination, and the patient is given cyclophosphamide with other lymphodepleting agents followed by infusion of the prepared T cells and IL-2. (2) Chimeric antigen receptor (CAR) T-cell therapy involves isolating T cells from peripheral blood and genetically modifying those cells with the CAR by using a viral vector or electroporation. The CAR domain consists of the scFV that recognizes the antigen, a spacer region, a transmembrane domain and a signaling domain. First-generation CARs contain only CD3ζ domain, whereas second- and third-generation CARs include additional costimulatory domains such as CD28, OX40, ICOS, 4-1BB and others to promote T-cell effector functions. (D, bottom left) NK cell and tumor interaction. Radiation increases expression of MHC-1 and stress-induced NK cell-stimulating ligands by tumor cells. The activating ligands bind to NKG2D on the NK cells to promote NK cell-mediated lysis of tumor cells; the MHC-1 on tumor cells binds KIR2DL on the NK cells to decrease NK-cell activity. Anti-inhibitory KIR antibodies (e.g., IPH2101) prevent MHC-1 signaling from silencing NK cells. Alternatively, NK cells can be delivered via donor hematopoietic stem cell transplantation or by isolation and ex vivo expansion.