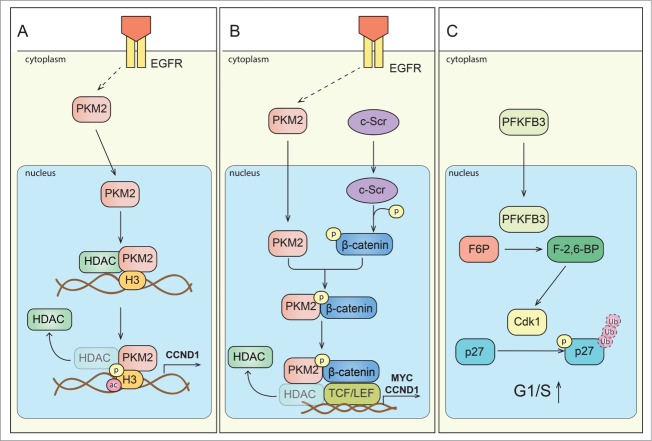
Figure 2.
Signaling of metabolic enzymes controls cell cycle progression. (A) Scheme depicting direct interaction of PKM2 and histone H3. Upon activation of epidermal growth factor receptor (EGFR), PKM2 translocates to the nucleus. PKM2 directly interacts with histone H3 and subsequently can phosphorylate it (P) at H3-T11, which leads to histone deacetylase 3 (HDAC3) removal from the promoter region of a.o. CCND1 (encoding cyclin D1 expression), histone H3-K9 acetylation (ac) and cyclin D1/Myc transcription. Expression of these genes is critical for G1/S phase transition. (B) Scheme depicting regulation of gene expression by PKM2 mediated β-catenin transactivation. Upon EGFR activation, PKM2 translocate to the nucleus, where it binds to c-Src-mediated phosphorylated β-catenin. Phosphorylated (P) β-catenin binds PKM2, and this interaction allows the protein complex to interact with transcription factor 4 (TCF4), and to bind to the promoter of target genes (CCND1, MYC) resulting in the dissociation of HDAC3 and activation of gene transcription. (C) Proposed scheme of PFKFB3 mediated effects on cell cycle regulators. PFKFB3 (splice variant 5) can localize to the nucleus, where its product, Fru-2,6-BP, activates cyclin-dependent kinase-1 (CDK1). CDK1-mediated phosphorylation (P) of p27, would then lead to p-p27 ubiquitination and subsequent degradation by the proteasome. These changes lead to acceleration of cell cycle progression at G1/S.