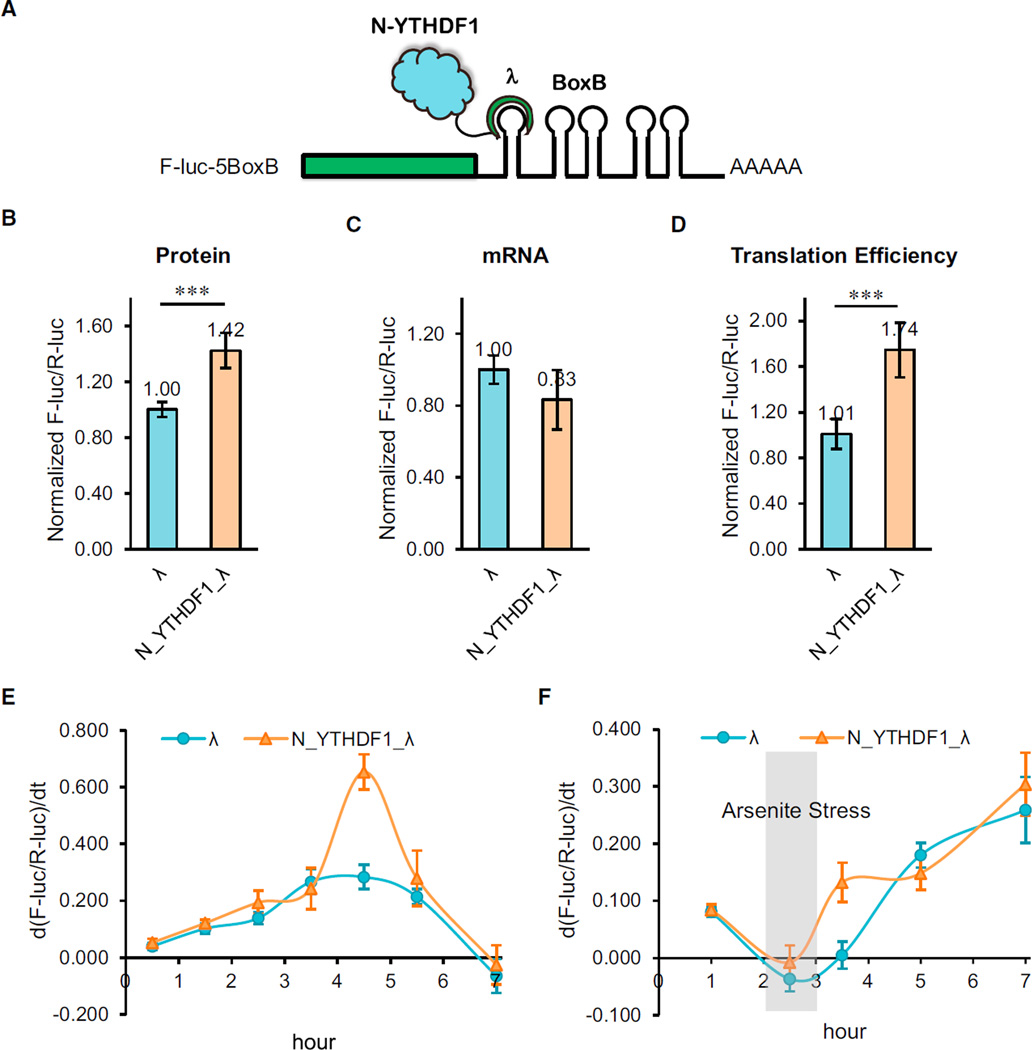
Figure 4. The N-Terminal Domain of YTHDF1 Promotes Protein Production in a Tethering Assay.
(A) Construct of the tethering reporter assay. The mRNA reporter consists of an inducible promoter, firefly luciferase as the coding region, and five Box B sequence at 3′ UTR (F-luc-5BoxB). The N-terminal domain of YTHDF1 (N_YTHDF1) was fused with λ peptide (N_YTHDF1_λ), which recognizes Box B RNA with a high affinity. R-luc lacks the inducible promoter and was used as an internal control to normalize the F-luc signal.
(B) Under constant induction, the tethering of N_YTHDF1_λ to F-luc-5BoxB led to an on-average 42% increased translation in comparison with the control. The translation outcome was determined as a relative signal of F-luc divided by R-luc. Error bars, mean ± SD, p = 5.9 × 10−4 (two-sided Student’s t test for paired samples), n = 6 (three biological replicates × two technical replicates).
(C) Under constant induction, the mRNA abundance decreased slightly in the N_YTHDF1_λ-tethered group compared with the control. The mRNA abundance was determined by qRT-PCR of F-luc and R-luc. Error bars, mean × SD, p = 0.031, n = 6.
(D) The translation efficiency of the reporter mRNA increased by ~72% in the N_YTHDF1_λ-tethered group compared with the control. The translation efficiency is defined as the quotient of reporter protein production (F-luc/R-luc) divided by mRNA abundance. Error bars, mean ± SD, p = 3.2 × 10−5, n = 6.
(E) F-luc-5BoxB was induced with a pulse expression for 2 hr. The mRNA reporter showed higher translation when tethered with N_YTHDF1_λ compared with the control. y axis, d(F-luc/R-luc)/dt, indicating the changing rate of protein production. Error bars, mean ± SD, n = 4.
(F) After a 2 hr pulse expression and a 1 hr arsenite (1 mM) stress treatment, translation of the reporter protein was largely diminished. The translation recovery was assessed after the stress was released. The result showed that the N_YTHDF1_λ-tethered group exhibited faster translation recovery than the control group. Error bars, mean ± SD, n = 4.