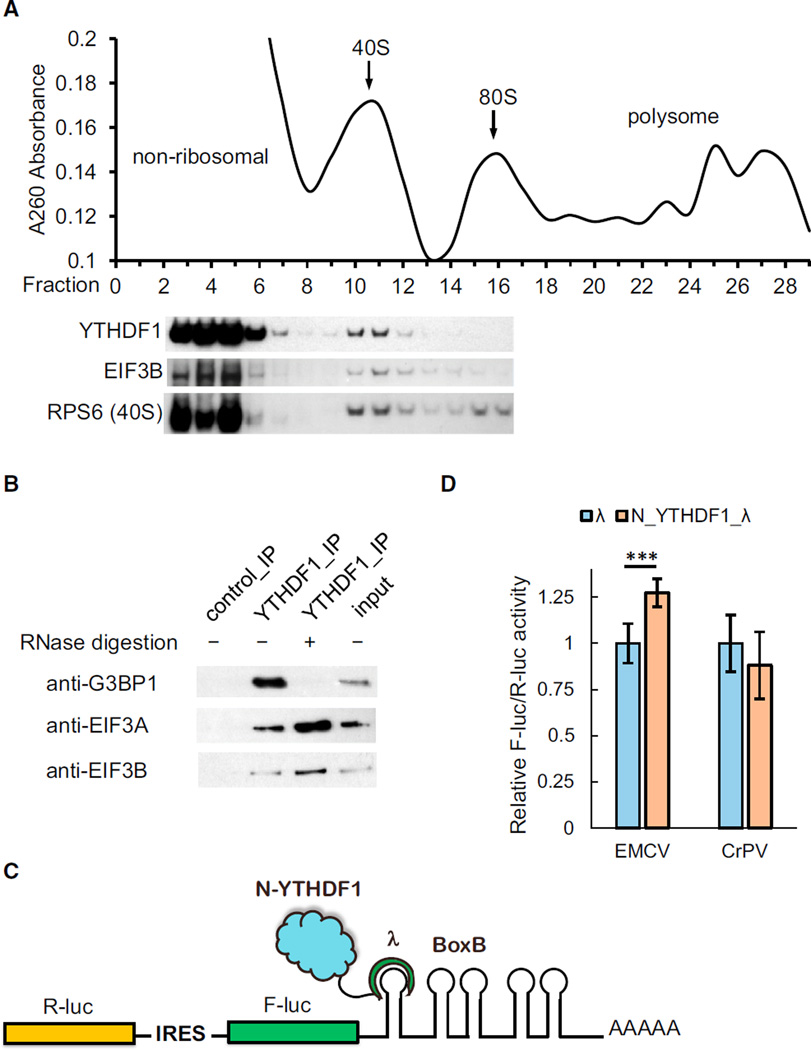
Figure 5. YTHDF1 Associates with Translation Initiation Factors, Ribosome, and Stress Granule Marker.
(A) Western blotting of Flag-tagged YTHDF1 on each fraction of 10%–50% sucrose gradient showing that YTHDF1 associates with ribosome. The fractions were grouped to non-ribosomal mRNPs, 40S–80S, and polysome. RPS6 is a protein subunit of 40S ribosome, and eIF3B is a component of the translation initiation complex (each lane is aligned to the corresponding fraction on the upper plot).
(B) Western blotting showing that eIF3A, eIF3B, and G3BP1 co-immunoprecipitated with YTHDF1. The association of YTHDF1 with G3BP1 requires RNA, and those with eIF3A/eIF3B are independent of RNA.
(C) Construct of the bicistronic IRES reporter assay. As the first coding region, R-luc reports cap-dependent translation of the mRNA and serves as control. The second coding region encodes F-luc whose translation is controlled by different types of upstream IRES elements. Five Box B sequence was inserted at the 3′ UTR as the tethering site for the N-terminal domain of YTHDF1.
(D) Tethering N_YTHDF1_λ to the EMCV IRES reporter led to an on-average 27% increased translation of F-luc compared with R-luc, whereas N_YTHDF1_λ had no effect on the CrPV IRES reporter. Error bars, mean ± SD, p (EMCV) = 9.5 × 10−5, P (CrPV) = 0.050, two-sided Student’s t test for paired samples, n = 8 (biological replicates).
See also Figures S4 and S5 and Table S2.