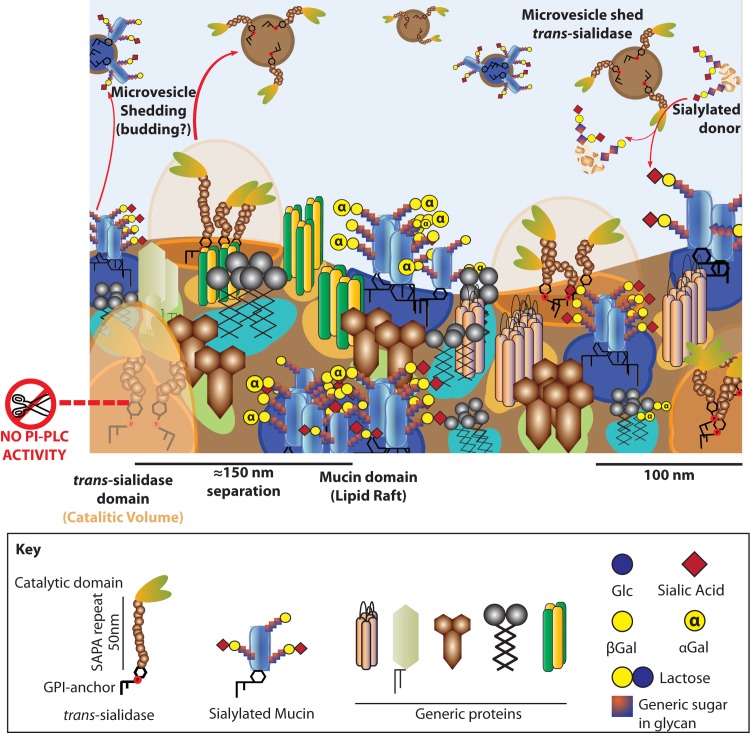
Fig 8. The membrane of trypomastigotes is complex to the nanometer scale.
Membrane model for T. cruzi trypomastigotes. The surface is packed in microdomains of different size, shape, lipid composition and embedded proteins. Some of these domains are detergent resistant, however this does not imply a functional profile. Mucins are included in DRMs whereas TS is not, thus being segregated in the membrane of trypomastigotes. This challenges the membrane bound TS as the sialylating factor for mucins, a role proposed for the shed TS instead. DRMs embed different proteins, many of them localized to the flagellum. Flagellum domains tend to be smaller and closer together than those in the cell body and suggest an association to the flagellar cytoskeleton. Mucins and TS are shed to the extracellular environment included in microvesicles probably resulting from membrane budding and fission events. Furthermore, TS is shed associated to vesicles instead of as a soluble protein. No hydrolysis of the GPI-anchors occurs in the trypomastigote stage.