Figure 1.
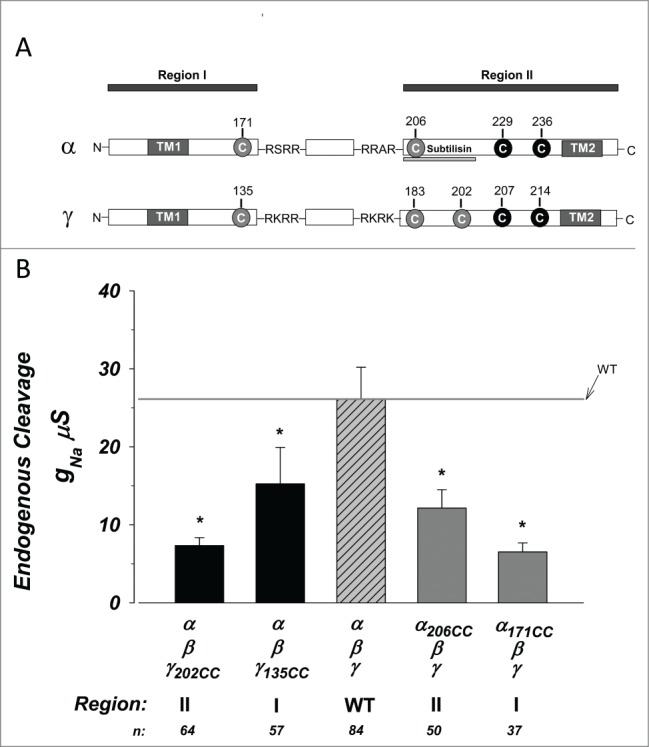
Exogenous cysteines bracketing the furin cleavage sites in α and Υ ENaC reduce baseline activity. Constructs were created containing cysteines bracketing the furin cleavage sites in α and γ ENaC. (A) Linear graphic representation of the sites of exogenous cysteines (gray circles) relative to the furin cleavage sites (4 sequence amino acids), as well as nearby endogenous cysteines (black circles). The subunits were divided into regions II and II based on the segment prior to the first cleavage and that following the second cleavage. The site cleaved by subtilisin is underlined. TM1 and TM2 indicate the 2 transmembrane domains. (B) Summary of the amiloride sensitive slope conductance at 0 mV. The magnitude of conductance was significantly lower in each construct compared to wild type control. * indicates P < 0.05. N = 37–84.
