Figure 2.
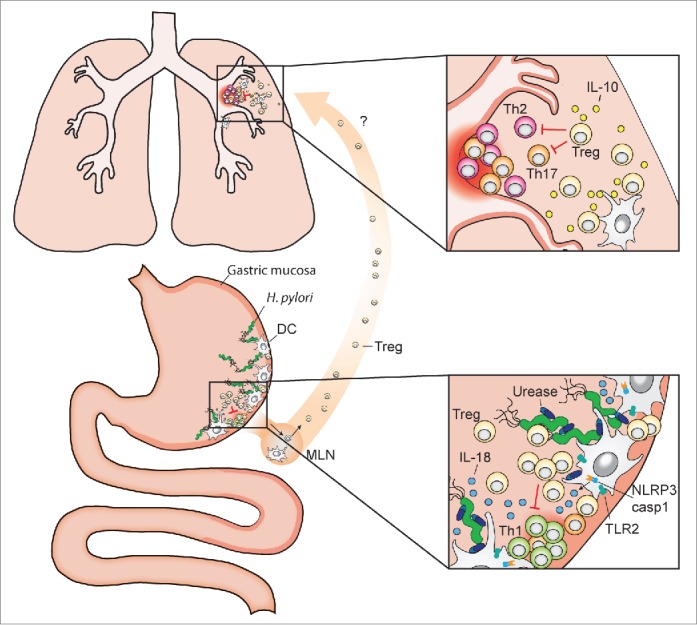
Systemic effects of gastric H. pylori colonization and inflammasome-dependent IL-18 production. The pathobiont H. pylori colonizes the gastric mucosa of roughly one half of the human population. Dendritic cells (DCs) and potentially other resident antigen-presenting cells sample H. pylori antigens and prime T-cell responses, either locally in the gastric mucosa and/or upon their migration to the draining mesenteric lymph nodes (MLN). DCs that have encountered urease-proficient H. pylori activate TLR2 signaling, the NLRP3 inflammasome and caspase-1 (lower inset), and process and secrete IL-18 (as well as IL-1β, not shown here), which promotes Treg differentiation. H. pylori-induced Tregs are required for the local suppression of anti-H. pylori T-effector cell responses and persistent H. pylori infection, and are also believed to migrate to the lung, where they prevent allergen-specific immune responses (upper inset). Treg- and DC-derived IL-10 contributes to H. pylori-specific allergy prevention in the lung.
