Figure 1. Establishment of an ubiquitin-independent cell-based assay for 26S proteasome inhibitors.
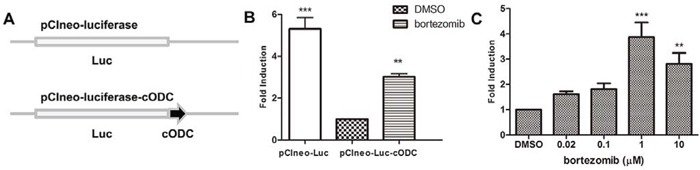
A. Schematic diagram of the luciferase reporter with or without the cODC motif. cODC is an ubiquitin-independent domain of ornithine decarboxylase that is required for 26S proteasome degradation. B. HEK293A cells were co-transfected in a 24-well plate with 0.1 μg of the pCIneo-luciferase or pCIneo-luciferase-cODC plasmids with 0.3 μg pSV-β-galactosidase expression plasmid. After 24 h of incubation, the cells were lysed and luciferase activity was measured and normalized to β-galactosidase activity. The results are expressed as relative-fold induction, referring to the ratio of normalized luciferase activity measured in the cells relative to the activity observed in the pCIneo-luciferase-cODC-transfected cells. C. HEK293A-luciferase-cODC cells were seeded in a 96-well plate and treated with various concentrations of bortezomib for 6 h. The cells were lysed and luciferase activity was measured. The results are expressed as relative-fold induction, referring to the ratio of normalized luciferase activity measured in bortezomib-treated cells relative to the activity observed in DMSO-treated cells.
