Abstract
Primary sequence motifs, with millimolar affinities for binding partners, are abundant in disordered protein regions. In multivalent interactions, such weak linear motifs can cooperate to recruit binding partners via avidity effects. If linear motifs recruit modifying enzymes, optimal placement of weak motifs may regulate access to modification sites. Weak motifs may thus exert stronger physiological relevance than suggested by their affinities, but molecular mechanisms of their function are still poorly understood. Herein, we use the N-terminal disordered region of the Hedgehog transcriptional regulator Gli3 (Gli31-90) to determine the role of weak motifs encoded in its primary sequence for the recruitment of its ubiquitin ligase CRL3SPOP and the subsequent effect on ubiquitination efficiency. The substrate adaptor SPOP binds linear motifs through its MATH domain and forms higher-order oligomers through its oligomerization domains, rendering SPOP multivalent for its substrates. Gli3 has multiple weak SPOP binding motifs. We map three such motifs in Gli31-90, the weakest of which has a millimolar dissociation constant. Multivalency of ligase and substrate for each other facilitates enhanced ligase recruitment and stimulates Gli31-90 ubiquitination in in vitro ubiquitination assays. We speculate that the weak motifs enable processivity through avidity effects and by providing steric access to lysine residues that are otherwise not prioritized for polyubiquitination. Weak motifs may generally be employed in multivalent systems to act as gatekeepers in posttranslational modification.
Keywords: multivalency, cancer, degron, Speckle-type POZ protein, NMR spectroscopy
Graphical Abstract

Introduction
Intrinsically disordered and flexible protein regions contain an abundance of primary sequence motifs that function to recruit binding partners, direct modifying enzymes and rewire signaling networks 1–8. Many of these linear motifs are only weakly conserved and may have affinities for their binding partners that are too weak to probe by high throughput methods. This raises the question of how strong an individual interaction must be to have a physiological function. If multiple linear motifs in a protein interact with repeats of interaction domains in a binding partner, described as so-called multivalent interactions, even weak individual interactions can contribute through avidity effects 9–14. Such multivalent interactions may thus serve to increase the affinity and specificity of modular interactions 15. In the context of substrate/enzyme interactions, multivalency can serve to regulate access to modification sites. Weak motifs may position an enzyme favorably relative to modification sites and thus contribute to the overall efficiency of the reaction to a higher degree than expected from their affinities. In multi-turnover reactions, avidity effects have the potential to reduce substrate off-rates and may thus serve to increase the processivity 16.
Many ubiquitin ligases recruit substrates by recognition of linear motifs (reviewed in 17). The existence of multiple weak motifs in substrates has been attributed to precise regulation of substrate levels, e.g. via the creation of switch-like dose response curves 18, 19. Some ubiquitin ligases oligomerize and form dimers, tetramers or even higher-order oligomers 20–25, 16. Abolishing their oligomerization often results in a decrease in their ubiquitination activity 14, 26, 27. The combination of an oligomeric ubiquitin ligase with a substrate with multiple weak motifs may create interesting opportunities for regulation.
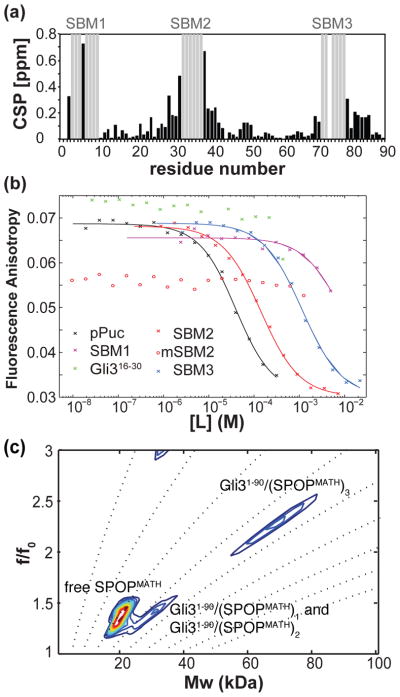
Herein, we characterize the function of weak linear motifs in a substrate for recruitment by the oligomeric substrate adaptor Speckle-type POZ protein (SPOP) of the cullin3-RING ligase (CRL3) 28–31. SPOP recognizes primary sequence motifs with the consensus sequence Φ-π-S-S/T-S/T (Φ, nonpolar residue; π, polar residue) in its substrates and binds them in the substrate-binding groove of its MATH (for meprin and TRAF homology) domain (Fig. 1a,b) 32. SPOP recruits additional CRL3 subunits through its 3-box 32 and dimerizes through its BTB (for Bric à Brac, Tramtrack and Broad-Complex) domain; crystal structures of these dimers are known (Fig. 1b) 32, 16. Recently, an additional oligomerization function has been assigned to the C-terminal BACK (for BTB and C-terminal Kelch) domain 16, 33 (Fig. 1a). The presence of 2 oligomerization domains facilitates self-association into higher-order SPOP homo-oligomers 16 (Fig. 1c), rendering SPOP multivalent for substrates. The SPOP substrates Gli2 and Gli3, which are transcriptional regulators of the Hedgehog signaling pathway, contain several regions that aid in their recognition by SPOP 34. SPOP and SPOP substrates are thus multivalent for each other. Single strong binding motifs for monomeric ubiquitin ligases can efficiently target substrates for degradation 17. The multivalency of SPOP/substrate pairs suggests an additional layer of regulation of substrate levels.
Figure 1. Multivalency of Gli3 and SPOP.
(a) Cartoon diagram of SPOP. SPOP contains a MATH domain for substrate recognition, a BTB domain for dimerization 32, and a BACK domain with an additional oligomerization function 16. We used constructs encoding monomeric SPOPMATH (28-166), dimeric SPOPMATH-BTB (28-337) 32, and higher-order oligomer-forming SPOPMATH-BTB-BACK (28-359) 16. (b) Ribbon diagram of the asymmetric SPOPMATH-BTB dimer bound to 2 SPOP binding motif peptides, shown in orange in stick representation, PDB ID 3HQI 32. One monomer in the ribbon diagram is colored more lightly. (c) Schematic of the domain structure of SPOP dimer (left) and SPOP oligomer (right). The linear SPOP oligomer schematic is based on the dimer structures of the BTB and BACK domains 33, 32 and indicates the possibility for indefinite self-association. (d) Gli3 has a folded zinc finger (Zn finger) DNA-binding domain that encompasses residues 455-636; all other regions are predicted to be largely intrinsically disordered (see Figure S1, Supplementary Material). By using the consensus SPOP binding sequence (Φ-π-S-S/T-S/T; Φ, nonpolar residue, π, polar residue) 32 and allowing for 1 mismatch, we predicted 29 weak consensus SB motifs, which are shown as red bars.
SPOP mutations have been recently identified in endometrial and colorectal cancers 35–37 among others, and SPOP is the most frequently mutated gene in prostate cancer 35. Most SPOP mutations are clustered in the substrate-binding groove and abolish substrate binding 35, 38, 39. Loss of SPOP expression occurs frequently in colorectal, gastric, and prostate tumors 40. Therefore, SPOP acts as a tumor suppressor under physiological conditions 41, and loss-of-function mutations or a redirection of SPOP activity to a different set of substrates serves to drive tumorigenesis 42, 43. Dissecting the mechanism of substrate recognition by SPOP is important to understand its normal function and role in pathogenesis.
In this study we used biophysical techniques and functional assays to characterize the role of weak SPOP binding motifs in a disordered region of Gli3 for recruitment of SPOP and for CRL3SPOP-mediated ubiquitination. We used nuclear magnetic resonance (NMR) spectroscopy, fluorescence anisotropy (FA) binding assays, and analytical ultracentrifugation (AUC) to map SPOP-binding (SB) motifs in a model multivalent substrate. We determined affinities to monomeric forms of SPOP by Biolayer interferometry (BLI) and assessed how SPOP oligomerization affects recognition of the multivalent substrate. In vitro ubiquitination assays provided insight into the role of multivalency and the contribution of individual weak motifs for polyubiquitination. These studies provide understanding of the functional role of weak SPOP binding motifs in a multivalent interaction.
Results
To gain molecular insights into the biophysical mechanism of the multivalent interaction between Gli3 and SPOP, we first analyzed the primary sequence of Gli3 and predicted 29 SB motifs based on the known SB consensus motif sequence Φ-π-S-S/T-S/T 32, if we allowed for 1 mismatched position (Fig. 1d). These motifs are likely solvent accessible; apart from the folded zinc finger DNA-binding domain (residues 455-636 according to sequence alignment with Gli1 44), Gli3 is predicted to be largely intrinsically disordered (see Supplementary Fig. S1). The interaction of a highly multivalent substrate with oligomeric SPOP is likely complicated. To gain insight into this interaction, we characterized the interaction of the 90-residue N-terminal Gli3 fragment (Gli31-90), which we predicted to have 4 SB motifs, with monomeric, dimeric and oligomeric variants of SPOP. We determined the position and affinities of SB motifs and how they contribute to SPOP recruitment and Gli3 ubiquitination.
A multivalent Gli3 fragment binds to the MATH domain substrate-binding groove
The MATH domain of SPOP (SPOPMATH) binds SB motifs in its groove across an antiparallel β-sheet (Figure 1b)32. To investigate whether multivalent substrates bind to SPOPMATH via the groove alone or whether they have additional interactions with other regions of SPOPMATH, we titrated Gli31-90 into 15N-labeled SPOPMATH and followed perturbations of SPOPMATH amide resonances by NMR (Fig. 2a). Resonance assignments of the free and bound states of SPOPMATH (Supplementary Fig. S2) allowed us to calculate chemical shift perturbations (Fig. 2b). Strong perturbations were observed only for residues clustered around the canonical groove (Fig. 2c). Residues known to directly contact the SB motif in crystal structures of SPOPMATH/motif complexes underwent the largest chemical shift perturbations. These perturbations were in slow exchange on the NMR time scale as observed by the progressive appearance of a bound-state signal while the signal of the free state disappeared (Supplementary Fig. S3). Specifically, residues Y87, F102, F125, K129, W131, F133 and K134, which are mutated in prostate cancer35, exhibited the largest perturbations. This result confirms the crucial role of these residues in recognizing substrates and supports previous findings that SPOPMATH mutants found in prostate cancer have substrate binding defects 38, 39. Binding of a peptide derived from the Drosophila melanogaster protein Puckered (pPuc, Ac91–ENLACDEVTSTTSSST106–NH2), which contains one known strong SB motif, produced similar chemical shift perturbations (Figs. 2d–f). Additional perturbations mapping to the back of SPOPMATH were small and observed for binding of both Gli31-90 and pPuc and therefore likely do not indicate additional interactions but rather small conformational changes of SPOPMATH upon ligand binding. These findings confirm that multivalent Gli31-90 interacts with SPOPMATH via its canonical substrate-binding groove.
Figure 2. Gli31-90 is recognized in the canonical MATH domain substrate-binding groove.
(a,d) 15N TROSY-HSQC spectra of free 15N SPOPMATH (black) and its complexes with (a) multivalent Gli31-90 (red) and (d) monovalent Puc91-106 (blue). (b, e) Combined 1H and 15N chemical shift perturbations of 15N SPOPMATH upon binding (b) Gli31-90 or (e) Puc91-106 demonstrate the involvement of the same interface. (c, f) Chemical shift perturbations mapped onto the surface of SPOPMATH in a color code from white to red (Gli31-90, c) or white to blue (Puc91-106, f) show that residues forming the canonical groove undergo the largest perturbations. Residues frequently mutated in prostate cancer cases are labeled 35.
Gli31-90 is intrinsically disordered
To map the location of SB motifs in the Gli31-90 sequence (Fig. 3a), we used NMR spectroscopy and first assigned the Gli31-90 resonances (Fig. 3b) from spectra recorded at 5° C where maximum chemical shift dispersion was observed. NMR spectroscopy is a powerful method for characterizing conformational and dynamic properties of IDPs 45–47. The narrow line widths and small 1HN chemical shift dispersion of the amide proton signals 48, 49 showed that this region of Gli3 is disordered, in agreement with in silico predictions (see Supplementary Fig. S1) and previous reports 50.
Figure 3. Gli31-90 is intrinsically disordered.
(a) Primary sequence of the Gli31-90 fragment. (b) 15N HSQC spectrum with resonance assignments, BMRB ID 26575. (c) 1H-15N hetNOE values at 25 °C, and (d) Secondary structure propensity (SSP) scores 53 calculated from Cα and Cβ chemical shifts demonstrate that Gli31-90 is intrinsically disordered and samples transient structure.
1H-15N heteronuclear NOE (hetNOE) values, which provide information on fast protein dynamics and flexibility 51, were mostly negative when measured at 600 MHz and 25 °C and slightly positive at the center of the protein at 800 MHz (Fig. 3c). The modulation of the hetNOE by the location in the polypeptide chain and the relatively strong modulation by the field strength was expected for an intrinsically disordered protein and indicated that the protein was flexible with some motional restriction 52. The region between resides 45 and 60, which exhibits the highest hetNOE values, also has higher transverse relaxation rates than the rest of the protein (Supplementary Fig. S4) suggesting the presence of transient interactions within this region.
Secondary structural propensities calculated from Cα and Cβ chemical shifts 53 demonstrated the presence of stretches with ≥10% helicity for Gli36-12, Gli318-29, Gli336-43, and Gli347-54 and up to 20% helicity for Gli377-86. The rest of the protein had a propensity to sample extended conformations (Fig. 3d). The disordered nature of Gli31-90 likely provides accessibility for SPOP to SB motifs for binding partners, including SPOP.
Gli31-90 has three weak SB motifs
In mapping the location of SB motifs in Gli31-90, we wanted to avoid bias towards the discovery of motifs with the known consensus motif, because weak binding motifs with distinct sequences may be able to contribute to the multivalent interaction with SPOP. We therefore implemented a three-pronged approach consisting of (1) a titration of SPOPMATH into 15N-labeled Gli31-90; (2) division of the candidate sequences into 15 residue-long peptides and assessment of their ability to compete with pPuc for SPOPMATH binding in a secondary fluorescence anisotropy (FA) binding assays; and (3) a tertiary NMR binding assay to monitor binding of peptides to 15N SPOPMATH. This combined approach allowed the identification of SB motifs and the exclusion of false- positive candidate motifs.
First, we titrated deuterated SPOPMATH into 15N-labeled Gli31-90 up to a 3.2-fold molar ratio and assigned the resonances of the complex. Resonances in three regions, at the N-terminus, in the middle and close to the C-terminus of the protein, were not assignable. We monitored chemical shift perturbations in 1H-15N heteronuclear single quantum coherence (HSQC) spectra. As expected, many resonances, in particular serine and threonine resonances (see Supplementary Fig. S5), experienced chemical shift perturbations because SB motifs are serine/threonine-rich. Chemical shift perturbations were largest around the regions that could not be assigned in the complex, suggesting that these regions contained SB motifs (Fig. 4a).
Figure 4. Gli31-90 can bind up to three SPOPMATH molecules.
(a) Chemical shift perturbations ((ΔH2 + 0.2ΔN2)1/2) for Gli31-90 upon SPOPMATH binding (at a molar ratio of 1:3.2). Residues shown in grey could not be assigned in the complex spectrum. These regions correspond to the three SPOP binding motifs in Gli31-90. (b) Peptides containing candidate SB motifs compete with fluorescein-Puc91-106 for binding to SPOPMATH in a fluorescence anisotropy competition assay. [L] is the peptide concentration. Solid lines are non-linear least squares fits to a complete competitive binding model 54. See Table 1 for KD values. (c) Two-dimensional size-and-shape distribution analysis of SV AUC data of a Gli31-90-SPOPMATH mixture. Shown are contour plots of c(M,f/f0) of the transformed velocity data with 0 fringes/S (white) to maximum value fringes/S (red), with increasing color temperature indicating higher values. The dotted lines are lines of constant s-value.
We then generated 15-residue-long peptides covering these regions and used a FA competition assay 54 to test their ability to bind to the SPOPMATH groove by competing with fluorescein-labeled pPuc (F-pPuc). The assay yielded a dissociation constant (KD) of 7.7 μM for pPuc (Table 1), which is in good agreement with previous data 32. Peptides encompassing regions Gli31-15, Gli330-45, and Gli370-84 competed with pPuc for binding to SPOPMATH and are referred to as SBM1, SBM2, and SBM3, respectively, hereafter (Fig. 4b and Table 1). We further confirmed the interaction of some of these peptides by monitoring chemical shift perturbations in 1H-15N HSQC spectra of 15N SPOPMATH. The peptides elicited similar perturbation patterns, but perturbations were weaker than those for pPuc or Gli31-90, suggesting individual motifs had weak binding affinities (see Supplementary Fig. S6).
Table 1.
Peptide affinities to SPOPMATH
| Motif | Residue No. | Sequence | KD (μM)a |
|---|---|---|---|
| pPuc | 91-106 | Ac-ENLACDEVTSTTSSST-NH2 | 7.7 ± 0.2 |
| SBM1 | 1-15 | Ac-MEAQSHSSTTTEKK-NH2 | ~4100 |
| Gli316-30 | 16-30 | Ac-VENSIVKCSTRTDVS-NH2 | nbb |
| SBM2 | 31-45 | Ac-EKAVASSTTSNEDES-NH2 | 80 ± 3 |
| mSBM2 | 31-45 | Ac-EKAVAGGSGSNEDES-NH2 | nbb |
| SBM3 | 71-84 | Ac-KVSEEPSTSSDERA-NH2 | 560 ± 30 |
| Gli341-59 | 41-59 | Ac-NEDESPGQTYHRERRNAIT-NH2 | nbb |
Errors represent standard errors from triplicate experiments from FA competition experiments.
No binding detected.
The binding affinities of SBM2 and SBM3 to SPOPMATH were weak, 80 μM and 560 μM, respectively (Table 1). As expected, mutation of the Ser/Thr-rich region in SBM2 to a generic disordered sequence (VASSTTS to VAGGSGS, previously used by Zhuang et al. to abolish substrate binding 32) decreased binding to below the detection limit of the assay. Although we were able to monitor the binding to SBM1 effectively by NMR, we estimated from FA that its KD was as high as ~4 mM. SBM1 lacks the hydrophobic residue at the motif N-terminus, probably accounting for its weak affinity. The order in which the signals at the resonance positions of the free state in individual motifs lost signal intensity in NMR titrations was consistent with the relative motif affinities measured for individual peptides by FA competition assays, indicating that transient structure in Gli31-90 did not alter the overall order of SB motif affinities (Supplementary Fig. 7).
The regions N- and C-terminal to SBM2 showed chemical shift perturbations as well, and so we tested peptides Gli316-30 and Gli341-59; neither peptide bound to SPOPMATH in the FA assay (Table 1, Figure 4b). Gli316-30 does not contain a conventional SB motif; it contains a cysteine in place of the serine that was previously suggested to be invariable (VKCST instead of Φ-π-S-S/T-S/T) 32. We speculated that this sequence might bind SPOPMATH, but did not observe consistent binding by FA- or NMR-detected titration (Fig. 4b and Supplementary Fig. S6). Gli341-59 does not contain an SB motif-like sequence. In the unbound state, this region of Gli31-90 has the highest hetNOE and R2 values (Fig. 3c and Supplementary Fig. S4), and therefore perturbation of its resonances may reflect a change in intramolecular interactions within Gli31-90 rather than intermolecular interactions with SPOPMATH 55, 56.
Together, these NMR and FA binding experiments show that we have identified 3 specific SB motifs in the N-terminus of Gli3. Among mammals, the entire N-terminus of Gli3 is highly conserved, while among vertebrates, the sequence similarity in the N- terminus is highest within the SB motifs (Supplementary Fig. S8), supporting the hypothesis that these motifs play a role in recruiting CRL3SPOP.
Gli31-90 can simultaneously bind up to three MATH domain molecules
To derive the stoichiometry of binding, we used sedimentation velocity (SV) AUC in combination with two-dimensional size-and-shape distribution analysis, which allows the determination of accurate molecular weights of several species with different frictional coefficients in a complex mixture 57. In the presence of an excess of SPOPMATH, up to 3 MATH domain molecules were able to bind to 1 Gli31-90 molecule (Fig. 4c). Furthermore, we observed both free SPOPMATH and a signal at higher molecular weights in the f/f0 value range of 1.2 to 1.6. We interpreted the latter to reflect a mixture of different 1:1 and 1:2 complexes, all likely having similar average molecular shapes and sizes. The 1:3 complex is easily distinguishable from the other signals because its larger Mw is combined with a large f/f0 value of 2–2.5, which we attribute to an extended and possibly rigid shape. SV analysis of two dilutions of the complex showed reduction of the fractional population of the largest complex as expected and confirms that the data are self-consistent (Supplementary Fig. S9). These data support the notion that Gli31-90 can interact with 1 SPOPMATH molecule through each of its 3 SB motifs demonstrating that they are accessible simultaneously.
Do SPOP dimers exhibit cooperative substrate binding?
Next, we explored whether the interaction of dimeric SPOP with substrates was dominated by avidity or cooperativity effects. We expected to find avidity effects in the SPOP/Gli31-90 interaction, i.e. the first SB motif/MATH interaction would result in an increased local concentration of additional interaction partners and thus enhance binding. However, the binding of ligands to oligomeric proteins is sometimes cooperative i.e., depends on the number of ligands already bound 58–60. A monovalent ligand cannot exhibit avidity toward dimeric SPOP, and can therefore be used to probe for cooperativity.
We used a direct FA binding assay in which we titrated either the SPOPMATH domain or the SPOPMATH-BTB dimer into the fluorescently labeled Puc peptide. Both interactions yielded the same KD of ~6 μM within error (Fig. 5). It is unlikely that the KD for the second MATH domain becomes too large to measure once the first MATH domain is bound, because a crystal structure of the SPOP dimer with 2 bound pPuc peptides exists 32 (see Fig. 1b). Therefore, the affinity of one MATH domain in the SPOP dimer is independent of the free or bound status of the other MATH domain. We conclude that the SPOP dimer does not exhibit macroscopic cooperativity toward monovalent substrates. Avidity effects may instead play a role in its interactions with multivalent substrates.
Figure 5. SPOP dimer binds monovalent substrate without cooperativity.
Direct fluorescence anisotropy binding data for monomeric SPOPMATH (black) and dimeric SPOPMATH-BTB (red) to monovalent Puc91-106. [P] indicates the protein concentration.
Multivalent interactions afford avidity
To test for the existence of avidity effects, we determined the apparent affinities of SPOP constructs with increasing multivalency, i.e. monomeric, dimeric and higher-order oligomeric SPOP, to Gli31-90. Higher-order oligomeric SPOP accesses a distribution of different oligomers depending on the protein concentration, though the dimer is always the predominant species (Marzahn, Mittag; unpublished data). We used a BLI assay in which we immobilized His-tagged Gli31-90 on the sensor and monitored binding of the three SPOP constructs to it. Their concentrations were normalized to the number of protomers in solution, i.e. solutions of monomeric, dimeric and oligomeric SPOP at the same concentration contained the same number of binding sites for SB motifs.
For SPOPMATH we obtained a KD of 21 μM (Table 2 and Supplementary Fig. S10). The increased apparent affinity, when compared with the highest microscopic affinity (80 μM for SBM2, Fig. 4b, Table 1), may be caused by (i) the high local concentration of motifs and therefore an increased probability for a MATH domain molecule to rebind after its release; (ii) an entropic advantage because of a greater number of different bound states in a multivalent substrate; or (iii) modifications of the affinities from flanking sequences outside of the core binding SB motifs (e.g. through long-range electrostatic interactions).
Table 2.
Gli31-90 affinities to SPOP constructs of different association state from kinetic fits of BLI data
| SPOP construct | association state | Kd (μM)a |
|---|---|---|
| SPOPMATH | monomer | 21±2 |
| SPOPMATH-BTB | dimer | 28±6 |
| SPOPMATH-BTB-BACK | higher-order oligomer | 1.5±0.1 |
Errors represent standard errors from triplicate experiments from BLI.
With dimeric SPOPMATH-BTB, we do not see a significant change in the affinity compared to the monomeric SPOPMATH. While we had expected an increased affinity due to engagement of two SB motifs via the two MATH domains, these data indicate that the SB motifs in Gli31-90 are not adequately spaced for avidity to the SPOP dimer. In contrast, the higher-order oligomer-forming SPOPMATH-BTB-BACK exhibited increased binding to Gli1-90 with a KD of 1.5 μM for Gli31-90 (Table 2). The multivalency of both proteins for each other thus led to an affinity increase due to avidity effects.
Contribution of weak SB motifs to ubiquitination
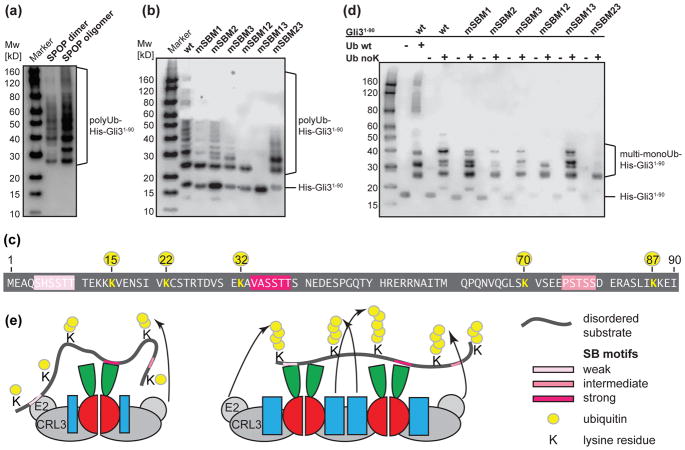
As we observed increased substrate binding by oligomeric SPOP, we wondered whether this would also lead to enhanced CRL3SPOP-mediated ubiquitination activity. We utilized His-Gli31-90 in standard in vitro ubiquitination assays with recombinant substrate, ubiquitin, E1, E2, and neddylated CRL3SPOP 32. Transfer of ubiquitin moieties onto lysine residues of His-Gli31-90 were monitored by Western Blot against the His-tag. A mixture of multi-mono- or poly-ubiquitinated species resulted in a regular laddering appearance (Fig. 6a, b, Supplementary Fig. S11). We compared CRL3SPOP activity in the context of dimeric SPOPMATH-BTB vs. oligomeric SPOPMATH-BTB-BACK. We observed efficient ubiquitination after a 20 min reaction for oligomeric SPOP but smaller ubiquitinated species with dimeric SPOP (Fig. 6a). These results are in agreement with previously reported enhancement of ubiquitination efficiency upon SPOP dimerization 32 and oligomerization 16. Together with our analysis of substrate binding, the data suggest that the multivalency of oligomeric SPOP and Gli31-90 for each other enhances recruitment of the substrate, thereby enhancing ubiquitination efficiency.
Figure 6. Weak SB motifs stimulate CRL3SPOP-mediated in vitro ubiquitination of Gli31-90.
(a) Western blot showing in vitro ubiquitination of His-Gli31-90 by neddylated CRLSPOP with dimeric SPOPMATH-BTB or oligomeric SPOPMATH-BTB-BACK. His-Gli31-90 is detected with an anti-6x-His antibody. (b, d) Western blot showing in vitro ubiquitination of wild-type (wt) and mutant His-Gli31-90 substrates by neddylated CRLSPOP with oligomeric SPOPMATH-BTB-BACK with (b) wt ubiquitin or (d) a lysine-less ubiquitin variant (Ub noK), which sustains only mono-ubiquitination on multiple lysines. mSBM1, mSBM2, mSBM3, and combinations refer to inactivating mutations of the 3 SB motifs. (c) Schematic representation of ubiquitin-modified lysine residues (yellow) in wt Gli31-90 substrate. (e) Model proposing the role of weak SB motifs and SPOP oligomerization in substrate recruitment and polyubiquitination. Dimeric SPOP recruits substrates with lower affinity and may not provide suitable steric access to lysine residues on growing polyubiquitin chains. In contrast, oligomeric CRLSPOP (a tetramer is shown for clarity) mediates enhanced recruitment via avidity effects and effective polyubiquitination through sterically favorable positioning relative to multiple catalytic centers in the oligomeric CRL. SB motifs are depicted as pink bars, the color saturation decreases for weaker motifs. Additional CRL components are not depicted for clarity.
We had observed a range of affinities for SB motif binding to SPOP and therefore postulated that the highly conserved motifs, even though weak, would play a distinct functional role. To test this hypothesis, we generated mutant Gli31-90 constructs that carried inactivating mutations 32 in 1, 2 or all 3 SB motifs. Strikingly, the mutation of SBM1, which had the weakest binding affinity, resulted in a substantial attenuation of ubiquitination (Fig. 6b). Mutation of SBM2, which had the strongest binding affinity and was expected to essentially abolish the recruitment of SPOP, only had a moderate effect on ubiquitination levels (Fig. 6b). In general, combinations of mutations led to increasing loss of ubiquitination. Interestingly, a Gli31-90 mutant that carried only SBM1 sustained relatively efficient ubiquitination. These results demonstrate that even weak motifs in Gli31-90 contribute to ubiquitination by CRL3SPOP. Reducing the multivalency, either by restricting the SPOP oligomerization state to dimers or by inactivating SB motifs in Gli31-90, reduced the overall ubiquitination efficiency.
Several studies have shown the importance of an optimal placement of acceptor lysines relative to the catalytic cysteine in ubiquitin ligases 61–64. We speculated that the unexpected loss of ubiquitination in the SBM1 mutant, and the lesser sensitivity of the SBM2 mutation, reflected the accessibility of modifiable lysine residues in Gli31-90. We characterized the lysine residues that were ubiquitinated in wt Gli31-90 by using tryptic digestion and liquid chromatography - tandem mass spectrometry (LC-MS/MS) analysis of reaction mixtures and identified 5 ubiquitinated lysine residues, K15, K22, K32, K70 and K87 (Fig. 6c for summary, Supplementary Fig. S12). We observed ubiquitination of the same lysine residues also for all mutants we tested, which included mSBM1, mSBM2, and mSBM23. While these experiments did not inform on the extent of ubiquitination at individual lysine residues, the results showed that not all SB motifs needed to be present simultaneously to provide access for lysine modification.
To further characterize whether the ubiquitination defect in some of the Gli3 mutants was a result of the modification of fewer lysine residues or less efficient building of polyubiquitin chains on all modifiable lysines, we next carried out ubiquitination reactions with a lysine-less ubiquitin mutant (Ub noK, in which all lysines are replaced with arginines). Ub noK does not support building of polyubiquitin chains but supports mono-ubiquitination at individual lysine residues. The reactions were carried out for 1 hr with higher E1 concentrations to reach an endpoint that showed the maximum number of modifiable lysine residues. We observed up to 5 modified lysine residues on wt Gli31-90 (Fig. 6d) in agreement with the LC-MS/MS results. Mutating one SB motif had only minor effects, with substrates still containing ~4 ubiquitinated lysines, although the ubiquitination patterns varied slightly among mutants, as observed by the bands on the Western blot (Fig. 6d). Most Gli31-90 mutants with less than 2 SB motifs were ubiquitinated on fewer lysines. The mSBM13 mutant, containing the highest affinity SBM2 motif, however, sustained ubiquitination on 4 lysine residues. The mSBM23 mutant, which contains only the weakest affinity SBM1 motif, was only modified on ~1–2 lysines. This is in contrast to the results with wt ubiquitin, which show that this mutant is still actively ubiquitinated (Fig 6b). Therefore, the weakest motif, SBM1, is required to promote efficient polyubiquitination of Gli31-90.
These results demonstrate that monoubiquitination is sustainable by the highest affinity motif, but that the presence of additional motifs, even if they have millimolar affinities, drives effective polyubiquitination, likely by enabling processivity of the ligase.
Discussion
Herein, we have demonstrated that weak linear binding motifs can strengthen protein/protein interactions and mediate function in multivalent systems, even if their dissociation constants are in the millimolar range. We identified three SB motifs in the intrinsically disordered N-terminus of Gli3; the strongest motif had a microscopic KD of 80 μM, while the others were in the hundreds of micromolar to millimolar range (Table 1). Together, they mediated binding to oligomeric SPOP at a low micromolar affinity (Table 2). These observations support a model in which dual multivalency, i.e. multivalency of each binding partner for the other, enhances binding through avidity effects, where the resulting binding is stronger than each individual interaction. The likely mechanism is the increase of the local concentration of a second pair of binding partners once the first interaction is made 11, 9. Interestingly, SPOP dimerization did not enhance binding of Gli31-90 compared to the SPOP monomer (Table 2). Although Gli31-90 supports binding of three MATH domain molecules simultaneously (Figure 4c), the SB motifs may not be optimally spaced for avidity with SPOP dimers.
In addition to the role of multivalency in substrate recruitment, multivalency was required for the full activity in an in vitro ubiquitination assay. Inactivating either SPOP’s ability to oligomerize or inactivating the weakest Gli31-90 SB motif led to a defect in Gli31-90 ubiquitination. However, all lysine residues that were ubiquitinated in wt Gli31-90 were also available for modification in the mutants. Therefore, we propose that the defect stems from a lack of processivity of CRL3SPOP, caused by the following two mechanisms: (1) Mutant substrates with lower affinities, due to a lack of avidity with SPOP, experience higher off-rates from the ligase and consequently lower ubiquitination rates; and (2) Binding to three SB motifs mediates sterically favorable placement of multiple catalytic ligase centers to accommodate the changing distances in a growing ubiquitin chain. We conclude that SBM1, the weakest binding motif, plays a particularly important role in positioning the substrate relative to catalytic cysteines because of its disproportionate effect on polyubiquitination. For mSBM23, the mutant that only harbors SBM1, monoubiquitination is reduced to 1 site (Fig. 6d), but polyubiquitination of this mutant is more effective than that for others (Fig. 6b). We speculate that SPOP oligomerization has a similar functional impact as adding additional weak SB motifs to a substrate, i.e. that multivalency of the substrate and CRL3SPOP for each other are decisive for processivity and thus polyubiquitination.
Our data suggest that even motifs that vary substantially from the consensus sequence support substrate ubiquitination, warranting a broader search for substrates than possible by sequence comparison with known motifs. How SPOP achieves substrate specificity despite its ability to recognize very weak motifs remains to be investigated.
Previous work showed that CRL3SPOP-mediated in vitro ubiquitination of Puc is driven by its strongest motif and that contribution of the 2 weaker motifs is negligible 32. The inactivity of the weak motifs in Puc might be explained by the use of dimeric SPOP in the previous report and the concurrent lack of dual multivalency and avidity. The absolute affinity of the motifs in Puc vs Gli31-90 also differ; the strong Puc motif is the strongest known SB motif with a KD of ~4 μM and may therefore dominate SPOP recruitment, even in the absence of SPOP oligomerization. We speculate that weak motifs are only functional in the context of highly multivalent substrates. They may serve to enable ultrasensitive substrate concentration/degradation responses 65. In contrast, we speculate that substrates with few strong motifs are polyubiquitinated constitutively because they are readily recruited to the ligase.
In addition to avidity, cooperativity is a possible mechanism for enhancing binding to oligomeric proteins. As cooperativity requires concerted changes of conformation or dynamics within the oligomeric protein upon binding the first binding partner, avidity may be simpler to realize through the use of a disordered protein with multiple motifs. The ubiquitous nature of disordered regions and linear motifs within them may attest to this fact.
Extrapolating from the 3 SB motifs we have mapped in Gli31-90, assuming constant density of SB motifs in all disordered regions, we would expect up to 46 SB motifs in full-length Gli3. Obviously, this deduction may not reflect reality; the density of SB motifs may be higher in the N-terminus. Nevertheless, previous reports have mapped several SPOP-recruiting regions in different parts of Gli3 34. In fact, a high density of short linear motifs has been reported in disordered regions of some proteins as a functional benefit and is therefore not unexpected66, 67. We expect that the presence of many weak motifs in combination with higher-order oligomerization of a binding partner enables tight regulation of the interaction. In fact, higher-order oligomerization of enzymes and the assembly of signaling machines are emerging as a new paradigm of signal transduction and are thought to mediate signal amplification, ultrasensitive responses, noise reduction, and exquisite temporal and spatial signaling control (reviewed in65).
The dysregulation of the Gli3/SPOP interaction may be expected to affect Gli3 turnover. Mutation of S8 and S36 in Gli3 have been reported in cancers (see www.cBioPortal.org, 68, 69) and may negatively affect SPOP recruitment because these serines are located in motifs characterized herein, SBM1 and SBM2 (Figure 3a, b). SB motifs in other parts of Gli3 not characterized here may be affected similarly. Though mutation of several SB motifs in a substrate is an unlikely event, single point mutations in SPOP can greatly affect its multivalency. Indeed, mutations of BTB and BACK domain interface residues that may affect SPOP’s ability to oligomerize have been found in sequencing efforts of melanoma and uterine cancers (see www.cBioPortal.org, 68–71). Mutations in the substrate binding groove of the MATH domain are usually not accompanied by loss of heterozygosity 35. In the presence of wt and mutant SPOP, SPOP hetero-oligomers with reduced multivalency must form, because wt protomers are able to bind SB motifs while mutant protomers are not. While levels of substrates with many weak linear motifs may be exquisitely regulated in healthy cells, in the presence of wt/mutant SPOP hetero-oligomers, their turnover is likely impacted more strongly than that of substrates with individual strong motifs. SPOP mutations may thus affect subsets of substrates, potentially changing substrate selectivity 43.
Despite tremendous progress in our understanding of individual systems, the rules of lysine selection by E2/E3/ubiquitin-like modifier trios 62, and for achieving monoubiquitination and polyubiquitination 72–74 are still poorly understood in general. The presence of multiple linear motifs have the potential to drive lysine selection and determine the extent of polyubiquitination as we suggest here; altering the number of ‘active’ linear motifs by posttranslational modification or conformational changes thus adds an additional possible regulatory layer. If the extent of ligase oligomerization is regulated as well, as has been suggested previously 16, astounding combinatorial possibilities for substrate selection ensue.
Weak linear motifs, when appearing in multiples in disordered protein regions, have the potential for extensive function. A deeper understanding of the regulation of CRL3SPOP activity toward multivalent substrates will ultimately provide insight into protein homeostasis in health and disease.
Methods
Constructs and cell cultures
The coding sequence for a Gli3 fragment spanning residues 1-90 (Gli31-90) was cloned into a pET28a vector and overexpressed in E. coli BL21 (DE3) RIPL cells (Agilent Technologies) in LB medium at 37°C with shaking at 280 rpm, and expression was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). The expression of GST-SPOPMATH (residues 28-166) and dimeric His-MBP-SPOPMATH-BTB (residues 28-337) has been previously described 32. Briefly, these constructs, GST-SPOPMATH-BTB and His-SUMO-SPOPMATH-BTB-BACK (residues 28-359) were expressed in BL21 GOLD cells (Agilent Technologies) in LB medium for unlabeled samples or M9 minimal medium supplemented with15 N NH4Cl and/or 13C glucose, depending on the desired labeling scheme. Expression was induced with 0.6 mM IPTG at OD600 ~ 0.6 at 18°C with shaking at 280 rpm overnight. Cells were harvested by centrifugation and lysed with a Microfluidizer (Microfluidics).
Protein purification
His-tagged Gli31-90 was purified using 20 mL Ni-NTA resin in buffer containing 50 mM Tris pH 8, 300 mM NaCl, 30 mM imidazole, and 2 mM β-mercaptoethanol (β-ME), and eluted in buffer containing 25 mM Tris pH 8, 300 mM NaCl, 300 mM imidazole, and 2 mM β-ME. The resulting protein was cleaved with tobacco etch virus (TEV) protease under dialysis at 4°C overnight against 25 mM Tris pH 8, 500 mM NaCl, 20 mM imidazole, and 5 mM β-ME, or the His-tag left for BLI and ubiquitination assays. The cleaved product was passed through Ni-NTA resin to remove uncleaved protein and TEV protease.
GST-SPOPMATH, GST-SPOPMATH-BTB and GST-SPOPMATH-BTB-BACK were purified using glutathione-Sepharose in PBS pH 7.3 and 5 mM DTT and eluted with PBS pH 7.3, 5 mM DTT, and 10 mM reduced L-glutathione. His-MBP-SPOPMATH-BTB was purified on 20 mL of Ni-NTA resin, followed by simultaneous TEV cleavage and dialysis at 4°C into PBS pH 7.6 and 5 mM DTT overnight. Ion exchange chromatography (IEX) to separate the cleaved tags from SPOP was performed on a 5 mL HiTrap SP column (GE Healthcare, for SPOPMATH) or a 5ml HiTrap Q column (for SPOPMATH-BTB), using a gradient from 0 to 700 mM NaCl.
All proteins were further purified by size exclusion chromatography on a HiLoad 16/60 Superdex 75 or 200 gel filtration column in 20 mM Tris pH 7.6, 150 mM NaCl, and 5 mM DTT or in NMR buffer (see below).
The protein purity was at least 95%, as shown by SDS-PAGE and SV AUC (see Supplementary Fig. S13). Protein identities were confirmed by both top-down and bottom up mass spectrometry.
Peptide synthesis
Fifteen residue peptides encompassing the SBMs were synthesized through the Hartwell Center, St. Jude Children’s Research Hospital, or purchased from GenScript. Each peptide allows for ~4 residues preceding and following the consensus sequence. All peptides were modified by N-terminal acetylation and C-terminal amidation.
Fluorescence anisotropy
An N-terminally fluorescently labeled Puc peptide (F-pPuc) with sequence 91Ac-ENLACDEVTSTTSSST-NH 107 2 was purchased from GenScript. For direct FA binding assays, increasing concentrations of SPOPMATH and SPOPMATH-BTB were titrated into 40 nM F-pPuc in a buffer containing 20 mM Tris pH 7.4, 150 mM NaCl, 1% bovine serum albumin (BSA, Sigma), and 0.01% Triton, and the FA monitored with an EnVision multilabel plate reader (PerkinElmer) at 25°C. For competition experiments, increasing concentrations of each peptide were individually titrated into a mixture of 6 μM SPOPMATH and 40 nM F-pPuc and the FA was monitored. Analysis was performed as described previously 54.
Nuclear magnetic resonance spectroscopy
NMR data were acquired on Bruker Avance 600 and 800 MHz spectrometers equipped with TCI triple-resonance cryogenic probes and pulsed-field gradient units. All samples were prepared in an NMR buffer consisting of 50 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, and 5 mM DTT pH 6.0 at 5°C. For assignment, a 1.5 mM 15N Gli31-90 sample was used to acquire standard triple-resonance backbone assignment experiments. These included a HNCACB and CBCA(CO)NH with 16 scans and 8 scans, respectively, and 1024 (1H) × 28 (15N) × 60 (13C) complex data points, with 10 ppm, 22 ppm, and 70 ppm for 1H, 15N and 13C sweep width, respectively. HNCO and HN(CA)CO were both acquired with 8 scans, 1024 (1H) × 20 (15N) × 64 (13C) complex data points, with 10 ppm, 22 ppm, and 22 ppm for 1H, 15N and 13C sweep widths, respectively.
Initial SPOPMATH assignments were obtained from the authors who solved the solution NMR structure of SPOPMATH (pdb: 2CR2) and the assignments were confirmed using 3D HNCA (1024 (1H) x 24 (15N) x 40 (13C) complex data points, with 14 ppm (1H), 32 ppm (15N) and 32 ppm (13C) sweep width) and CBCA(CO)NH (1024 (1H) x 24 (15N) x 44 (13C) complex data points, with 14 ppm (1H), 32 ppm (15N) and 72 ppm (13C) sweep width) experiments measured with 8 and 16 scans, respectively. Backbone resonances of residues E46, K74, G75, S96, S119-Y123 were not observed in the spectrum and therefore were unassigned. Since the binding pocket residues exhibited slow exchange in the SPOPMATH-Puc (1:5) complex, this spectrum was reassigned using 3D HNCA, HNCO, HN(CA)CO and HNCACB experiments. For all of the 3D experiments the data were collected at 298 K for samples containing 0.5 mM SPOPMATH in NMR buffer. The positions of the resonances in the binding pocket that were in slow exchange in SPOPMATH-Gli31-90 (1:2) and SPOPMATH-SBM2 (1:4) complexes were very close to that of SPOPMATH-Puc complex and thus were assigned as for the SPOPMATH-Puc complex. Data were processed using BRUKER Topspin version 3.2, NMRPipe (v.7.9) 75 and analyzed using CARA (v.1.8.4) 76.
1H,15N TROSY-HSQC spectra of 350 μM 15N SPOPMATH in NMR buffer with increasing concentrations of Gli31-90 (to molar ratios of 1:1.75) were collected with 1024 (1H) × 90 (15N) complex data points and 32 scans with 12 ppm and 29 ppm for 1H and 15N sweep widths, respectively. Similar spectra were recorded for the complexes in the presence of different peptides, with protein/peptide molar ratios of 1:5 for Puc, 1:8 for Gli316-30, 1:4 for SBM2, and 1:4 for SBM3.
Gradient-selected sensitivity-enhanced 1H,15N HSQC spectra of 400 μM 15N Gli31-90 in NMR buffer with increasing concentrations of SPOPMATH (to molar ratios of 1:3) were recorded with 1024 × 150 complex data points and 16 scans. Intensity ratios for each resonance were calculated from the intensities in the free state and from intensities at increasing molar ratios at the same resonance frequencies to combine chemical shift perturbations and broadening into a single parameter of intensity loss. hetNOEs were collected with a 2-sec relaxation delay and with and without a 5-sec presaturation delay, using 1024 × 200 complex data points and 32 scans with 10 ppm, and 24 ppm for 1H, and 15N sweep widths, respectively. The hetNOE values were calculated from the ratio of peak intensities of the saturated and unsaturated spectra. All the spectra were referenced directly using DSS for the 1H dimension and 13C and 15N frequencies were referenced indirectly.
Secondary structural propensities were calculated by using 13Cα and 13Cβ chemical shifts using the SSP program 53.
Analytical ultracentrifugation
Sedimentation velocity experiments of all protein samples were conducted in a ProteomeLab XL-I analytical ultracentrifuge (Beckman Coulter, Indianapolis, IN) following standard protocols unless mentioned otherwise 57, 77. A mixture of Gli31-90 (156 μM)/SPOPMATH (525 μM) and a dilution series thereof, in the ultracentrifugation buffer (100 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, and 5 mM DTT at pH 6.0) was loaded into a cell assembly comprised of a double sector charcoal-filled centerpiece with a 12 mm path length and sapphire windows. The cell assembly, containing identical sample and reference buffer volumes of 400 μL, was placed in a rotor and temperature equilibrated at rest at 20 °C for 2 hours before it was accelerated from 0 to 50,000 rpm. Rayleigh interference optical data were collected continuously for 10 hours. The velocity data were modeled with diffusion-deconvoluted sedimentation coefficient distributions c(s) in SEDFIT (https://sedfitsedphat.nibib.nih.gov/software/default.aspx), using algebraic noise decomposition and with the signal-average frictional ratio and meniscus position refined with non-linear regression. Maximum entropy regularization was applied at a confidence level of P-0.70. A two-dimensional size-and-shape distribution model, c(s,f/f0) (with the one dimension the s-distribution and the other the f/f0-distribution) was also calculated with the same interference fringe displaced velocity data. The equidistant f/f0-grid was from 1.0 to 3.0 with 0.13 steps, the linear s-grid from 0.5 to 5 S with 100 s-values, and Tikhonov-Phillips regularization at one standard deviation was applied. The data were transformed to a c(M,f/f0) contour distribution plot with M the molecular mass and f/f0 the frictional ratio. The dotted lines indicate lines of constant s-value. The distributions were not normalized 57, 78.
BLI
Binding affinities of Gli31-90 to SPOPMATH, SPOPMATH-BTB, and SPOPMATH-BTB-BACK were measured by BLI using an OctetRED instrument (ForteBio). All proteins used for BLI were buffer exchanged with 20 mM HEPES, 150 mM NaCl, 5mM DTT, 0.005% Tween 20, pH 7.4. N-terminal His6-Gli31-90 (0.1 μM) was immobilized on Ni-NTA biosensor (ForteBio) for 300 seconds. For baseline and surface stability, the captured His6-Gli31-90 was cross-linked for 60 seconds with a mixture of 0.1 M ethyl(dimethylaminopropyl) carbodiimide (EDC) and 0.025 M N-Hydroxysuccinimide (NHS) and subsequently quenched with 1 M ethanolamine for 60 seconds. Biosensors with immobilized His6-Gli31-90 were subsequently dipped into different concentrations of SPOP samples for association until the signal reached a plateau. Binding of SPOPMATH-BTB-BACK was performed at concentrations of 0, 0.01, 0.05, 0.1, 0.5, 1, 2.5 and 5 μM, binding of SPOPMATH-BTB and SPOPMATH was performed at concentrations of 0, 0.1, 0.5, 1, 2.5, 5, 10 and 20 μM. The concentrations of SPOP constructs with different valency were normalized to the number of protomers in solution, i.e. solutions of 1 μM monomeric, dimeric and oligomeric SPOP contained the same number of binding sites for SB motifs. Dissociation of SPOP was monitored by subsequently incubating the biosensors in the buffer (20 mM HEPES, 150 mM NaCl, 5mM DTT, 0.005% Tween 20, pH 7.4). The sensorgram at each SPOP concentration was background subtracted using a reference sensorgram (obtained from biosensors without immobilized Gli31-90). The dissociation constants were obtained by fitting the data to a kinetic model for one site binding.
In vitro ubiquitination assay
Gli31-90 ubiquitination was sampled in 50 mM Tris pH 7.5, 150 mM NaCl, 10 mM MgCl2, 5 mM ATP, 1 mM DTT, and 2 mg/mL BSA at room temperature at time points from 0 to 20 min. The reaction mixture contained ubiquitinating enzymes at final concentrations of 0.25 μM UBA1 (E1; in assays with lysine-less ubiquitin 1 μM UBA1 to reach maximum ubiquitination more rapidly), 5 μM UbcH5B (E2), 5 μM NEDD8-CUL3-Rbx1 (E3), 5 μM SPOP (either SPOPMATH-BTB-BACK or SPOPMATH-BTB as substrate adaptor), 75 μM ubiquitin or a lysine-less ubiquitin mutant (both BostonBiochem) and 5 μM His6-tagged Gli31-90 or mutant versions. E1, E2, and E3 were purified as described previously 79, 80, 16. The substrate and products were visualized by Western blot with anti-His antibody. The mutant versions of Gli31-90 were obtained by site-directed mutagenesis by replacing the Ser/Thr-rich regions with the generic disordered sequence GGSGS.
Mass spectrometry analysis of ubiquitination patterns
Ubiquitination reactions were run on an SDS PAGE gel, and proteins in each lane were excised, reduced with DTT to break disulfide bonds and Cys residues were alkylated by iodoacetamide to allow the recovery of Cys-containing peptides. The gel bands were washed, dried in a vacuum concentrator and rehydrated with a buffer containing trypsin. Samples were digested overnight and the peptides were extracted using acetonitrile. The extracts were dried and each sample was then resuspended in 5% formic acid. For MS analysis, the dried peptides were analyzed on an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific) after separation on a 40cm X 75 μm id column packed with 1.9 μm C18 resin (Dr. Maisch GmbH, Germany). Separation was achieved by applying 10–40% buffer B gradient in 2 h (buffer A: 0.2% formic acid; buffer B: buffer A plus 70% ACN). The column was heated at 65°C by a butterfly portfolio heater (Phoenix S&T) to reduce backpressure. The mass spectrometer was operated in data-dependent and targeted mode with a survey scan in Orbitrap (60 000 resolution, 2 X 105 AGC target and 50 ms maximal ion time). During data dependent acquisition, Orbitrap survey spectra were scheduled for execution at least every 3 s, with the embedded control system determining the number of MS/MS acquisitions executed during this period. A list of masses corresponding to all putative lysine modification sites of the Gli31-90 variants was used for targeted MS/MS acquisition to increase the sensitivity of detection. The parameters for MS/MS scans were HCD, 1 X 105 AGC target, 128 ms maximal ion time, 0.4 m/z isolation window, 38 normalized collision energy, and 20 s dynamic exclusion. MS/MS raw files were converted into mzXML format and searched against Uniprot mouse database populated with user added protein sequences representing the mutated proteins by JUMP algorithm 81, a tag-based database search program.
Supplementary Material
Research Highlights.
Disordered proteins contain abundant weak motifs with unclear functional relevance.
We identify several weak SPOP binding motifs in the disordered N-terminus of Gli3.
These motifs cooperate in recruiting oligomeric SPOP through avidity effects.
A motif with a millimolar dissociation constant stimulates polyubiquitination.
Weak motifs may facilitate post-translational modification through positioning of enzymes.
Acknowledgments
We thank Jill Bouchard for technical help. This work was supported by R01GM112846- 01 and a V Foundation Scholar Award (to T.M.), the National Cancer Institute Cancer Center Support Grant P30CA21765 (at St. Jude Children’s Research Hospital), and the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations
- AUC
analytical ultracentrifugation
- BACK
BTB and C-terminal Kelch
- β-ME
β-mercaptoethanol
- BLI
Biolayer interferometry
- BTB, and Bric à Brac
Tramtrack and Broad-Complex
- CRL3
cullin3-RING ligase
- CRL3SPOP
CRL3 with substrate adaptor SPOP
- F-pPuc
fluorescently labeled Puc peptide
- FA
fluorescence anisotropy
- Gli31-90
90-residue N-terminal Gli3 fragment
- hetNOE
1H-15N heteronuclear NOE
- HSQC
heteronuclear single quantum coherence
- KD
dissociation constant
- LC-MS/MS
liquid chromatography - tandem mass spectrometry
- MATH
meprin and TRAF homology
- NMR
nuclear magnetic resonance
- pPuc
peptide derived from Puckered SB motif
- SB
SPOP-binding
- SPOP
Speckle-type POZ protein
- SPOPMATH
MATH domain of SPOP
- SPOPMATH-BTB
SPOP construct encompassing residues 28-337
- SPOPMATH-BTB-BACK
SPOP construct encompassing residues 28-359
- SV
sedimentation velocity
- TEV
tobacco etch virus
- Ub noK
lysine-less ubiquitin mutant
- wt
wildtype
Footnotes
Author Contributions
W.K.P., M.R.M., B.S. and T.M. designed the research; W.K.P., G.R., J.L., A.N., M.R.M., E.W., and A.A.H. performed the research; W.K.P., G.R., A.N., M.R.M., A.A.H., J.P. and T.M. analyzed data; and W.K.P. and T.M. wrote the paper; all authors edited the paper.
Conflict of Interest
The authors declare no conflict of interest.
Accession numbers
The backbone chemical shift assignments of SPOPMATH, SPOPMATH+Puc, and Gli31-90 were deposited in the BMRB database with the following IDs: 26629, 26631, and 26575.
References
- 1.Neduva V, Russell RB. Linear motifs: evolutionary interaction switches. FEBS Lett. 2005;579:3342–3345. doi: 10.1016/j.febslet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Mohan A, Oldfield CJ, Radivojac P, Vacic V, Cortese MS, Dunker AK, et al. Analysis of molecular recognition features (MoRFs) J Mol Biol. 2006;362:1043–1059. doi: 10.1016/j.jmb.2006.07.087. [DOI] [PubMed] [Google Scholar]
- 3.Fuxreiter M, Tompa P, Simon I. Local structural disorder imparts plasticity on linear motifs. Bioinformatics. 2007;23:950–956. doi: 10.1093/bioinformatics/btm035. [DOI] [PubMed] [Google Scholar]
- 4.Davey NE, Trave G, Gibson TJ. How viruses hijack cell regulation. Trends Biochem Sci. 2011;36:159–169. doi: 10.1016/j.tibs.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Edwards RJ, Davey NE, O’Brien K, Shields DC. Interactome-wide prediction of short, disordered protein interaction motifs in humans. Mol Biosyst. 2012;8:282–295. doi: 10.1039/c1mb05212h. [DOI] [PubMed] [Google Scholar]
- 6.Buljan M, Chalancon G, Dunker AK, Bateman A, Balaji S, Fuxreiter M, et al. Alternative splicing of intrinsically disordered regions and rewiring of protein interactions. Curr Opin Struct Biol. 2013;23:443–450. doi: 10.1016/j.sbi.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Tompa P, Davey NE, Gibson TJ, Babu MM. A million peptide motifs for the molecular biologist. Mol Cell. 2014;55:161–169. doi: 10.1016/j.molcel.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 8.Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol. 2015;16:18–29. doi: 10.1038/nrm3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz-Linek U, Pilka ES, Pickford AR, Kim JH, Hook M, Campbell ID, et al. High affinity streptococcal binding to human fibronectin requires specific recognition of sequential F1 modules. J Biol Chem. 2004;279:39017–39025. doi: 10.1074/jbc.M405083200. [DOI] [PubMed] [Google Scholar]
- 10.Hall J, Karplus PA, Barbar E. Multivalency in the assembly of intrinsically disordered Dynein intermediate chain. J Biol Chem. 2009;284:33115–33121. doi: 10.1074/jbc.M109.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi A, Goldstein B, Gnanakaran S. Quantifying intramolecular binding in multivalent interactions: a structure-based synergistic study on Grb2-Sos1 complex. PLoS computational biology. 2011;7:e1002192. doi: 10.1371/journal.pcbi.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan JL, Song Y, Barbar E. Structural dynamics and multiregion interactions in dynein-dynactin recognition. J Biol Chem. 2011;286:39349–39359. doi: 10.1074/jbc.M111.296277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyarko A, Song Y, Novacek J, Zidek L, Barbar E. Multiple recognition motifs in nucleoporin Nup159 provide a stable and rigid Nup159-Dyn2 assembly. J Biol Chem. 2013;288:2614–2622. doi: 10.1074/jbc.M112.432831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welcker M, Larimore EA, Swanger J, Bengoechea-Alonso MT, Grim JE, Ericsson J, et al. Fbw7 dimerization determines the specificity and robustness of substrate degradation. Genes Dev. 2013;27:2531–2536. doi: 10.1101/gad.229195.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbar E, Nyarko A. Polybivalency and disordered proteins in ordering macromolecular assemblies. Semin Cell Dev Biol. 2015;37:20–25. doi: 10.1016/j.semcdb.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Errington WJ, Khan MQ, Bueler SA, Rubinstein JL, Chakrabartty A, Prive GG. Adaptor protein self-assembly drives the control of a cullin-RING ubiquitin ligase. Structure. 2012;20:1141–1153. doi: 10.1016/j.str.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin- proteasome system. Nat Rev Mol Cell Biol. 2008;9:679–690. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang X, Orlicky S, Mittag T, Csizmok V, Pawson T, Forman-Kay JD, et al. Composite low affinity interactions dictate recognition of the cyclin-dependent kinase inhibitor Sic1 by the SCFCdc4 ubiquitin ligase. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3287–3292. doi: 10.1073/pnas.1116455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, et al. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature. 2001;414:514–521. doi: 10.1038/35107009. [DOI] [PubMed] [Google Scholar]
- 20.Ronchi VP, Klein JM, Edwards DJ, Haas AL. The active form of E6- associated protein (E6AP)/UBE3A ubiquitin ligase is an oligomer. J Biol Chem. 2014;289:1033–1048. doi: 10.1074/jbc.M113.517805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kentsis A, Gordon RE, Borden KL. Self-assembly properties of a model RING domain. Proc Natl Acad Sci U S A. 2002;99:667–672. doi: 10.1073/pnas.012317299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem. 2006;281:24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 23.Yin Q, Lin SC, Lamothe B, Lu M, Lo YC, Hura G, et al. E2 interaction and dimerization in the crystal structure of TRAF6. Nat Struct Mol Biol. 2009;16:658–666. doi: 10.1038/nsmb.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peschard P, Kozlov G, Lin T, Mirza IA, Berghuis AM, Lipkowitz S, et al. Structural basis for ubiquitin-mediated dimerization and activation of the ubiquitin protein ligase Cbl-b. Mol Cell. 2007;27:474–485. doi: 10.1016/j.molcel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Welcker M, Clurman BE. Fbw7/hCDC4 dimerization regulates its substrate interactions. Cell Div. 2007;2:7. doi: 10.1186/1747-1028-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poyurovsky MV, Priest C, Kentsis A, Borden KL, Pan ZQ, Pavletich N, et al. The Mdm2 RING domain C-terminus is required for supramolecular assembly and ubiquitin ligase activity. EMBO J. 2007;26:90–101. doi: 10.1038/sj.emboj.7601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang X, Orlicky S, Lin Z, Willems A, Neculai D, Ceccarelli D, et al. Suprafacial orientation of the SCFCdc4 dimer accommodates multiple geometries for substrate ubiquitination. Cell. 2007;129:1165–1176. doi: 10.1016/j.cell.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Munoz I, Lund AH, van der Stoop P, Boutsma E, Muijrers I, Verhoeven E, et al. Stable X chromosome inactivation involves the PRC1 Polycomb complex and requires histone MACROH2A1 and the CULLIN3/SPOP ubiquitin E3 ligase. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7635–7640. doi: 10.1073/pnas.0408918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon JE, La M, Oh KH, Oh YM, Kim GR, Seol JH, et al. BTB domain- containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. The Journal of biological chemistry. 2006;281:12664–12672. doi: 10.1074/jbc.M600204200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Zhang L, Wang B, Ou CY, Chien CT, Jiang J. A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Developmental cell. 2006;10:719–729. doi: 10.1016/j.devcel.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Ghanim M, Xue L, Brown CD, Iossifov I, Angeletti C, et al. Analysis of Drosophila segmentation network identifies a JNK pathway factor overexpressed in kidney cancer. Science. 2009;323:1218–1222. doi: 10.1126/science.1157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuang M, Calabrese MF, Liu J, Waddell MB, Nourse A, Hammel M, et al. Structures of SPOP-substrate complexes: insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Molecular cell. 2009;36:39–50. doi: 10.1016/j.molcel.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Geersdaele LK, Stead MA, Harrison CM, Carr SB, Close HJ, Rosbrook GO, et al. Structural basis of high-order oligomerization of the cullin-3 adaptor SPOP. Acta crystallographica. Section D, Biological crystallography. 2013;69:1677–1684. doi: 10.1107/S0907444913012687. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q, Shi Q, Chen Y, Yue T, Li S, Wang B, et al. Multiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21191–21196. doi: 10.1073/pnas.0912008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature genetics. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Gallo M, O’Hara AJ, Rudd ML, Urick ME, Hansen NF, O’Neil NJ, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet. 2012;44:1310–1315. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MS, Je EM, Oh JE, Yoo NJ, Lee SH. Mutational and expressional analyses of SPOP, a candidate tumor suppressor gene, in prostate, gastric and colorectal cancers. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2013;121:626–633. doi: 10.1111/apm.12030. [DOI] [PubMed] [Google Scholar]
- 38.Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, et al. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6997–7002. doi: 10.1073/pnas.1304502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An J, Wang C, Deng Y, Yu L, Huang H. Destruction of Full-Length Androgen Receptor by Wild-Type SPOP, but Not Prostate-Cancer-Associated Mutants. Cell reports. 2014;6:657–669. doi: 10.1016/j.celrep.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MS, Je EM, Oh JE, Yoo NJ, Lee SH. Mutational and expressional analyses of SPOP, a candidate tumor suppressor gene, in prostate, gastric and colorectal cancers. APMIS. 2013;121:626–633. doi: 10.1111/apm.12030. [DOI] [PubMed] [Google Scholar]
- 41.Davoli T, Xu AW, Mengwasser KE, Sack LM, Yoon JC, Park PJ, et al. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell. 2013;155:948–962. doi: 10.1016/j.cell.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G, Ci W, Karmakar S, Chen K, Dhar R, Fan Z, et al. SPOP promotes tumorigenesis by acting as a key regulatory hub in kidney cancer. Cancer Cell. 2014;25:455–468. doi: 10.1016/j.ccr.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theurillat JP, Udeshi ND, Errington WJ, Svinkina T, Baca SC, Pop M, et al. Prostate cancer. Ubiquitylome analysis identifies dysregulation of effector substrates in SPOP- mutant prostate cancer. Science. 2014;346:85–89. doi: 10.1126/science.1250255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavletich NP, Pabo CO. Crystal structure of a five-finger GLI-DNA complex: new perspectives on zinc fingers. Science. 1993;261:1701–1707. doi: 10.1126/science.8378770. [DOI] [PubMed] [Google Scholar]
- 45.Eliezer D. Biophysical characterization of intrinsically disordered proteins. Curr Opin Struct Biol. 2009;19:23–30. doi: 10.1016/j.sbi.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyson HJ, Wright PE. Nuclear magnetic resonance methods for elucidation of structure and dynamics in disordered states. Methods Enzymol. 2001;339:258–270. doi: 10.1016/s0076-6879(01)39317-5. [DOI] [PubMed] [Google Scholar]
- 47.Mittag T, Forman-Kay JD. Atomic-level characterization of disordered protein ensembles. Curr Opin Struct Biol. 2007;17:3–14. doi: 10.1016/j.sbi.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Zhang O, Kay LE, Olivier JP, Forman-Kay JD. Backbone 1H and 15N resonance assignments of the N-terminal SH3 domain of drk in folded and unfolded states using enhanced-sensitivity pulsed field gradient NMR techniques. J Biomol NMR. 1994;4:845–858. doi: 10.1007/BF00398413. [DOI] [PubMed] [Google Scholar]
- 49.Yao J, Dyson HJ, Wright PE. Chemical shift dispersion and secondary structure prediction in unfolded and partly folded proteins. FEBS letters. 1997;419:285–289. doi: 10.1016/s0014-5793(97)01474-9. [DOI] [PubMed] [Google Scholar]
- 50.Tsanev R, Vanatalu K, Jarvet J, Tanner R, Laur K, Tiigimagi P, et al. The transcriptional repressor domain of Gli3 is intrinsically disordered. PLoS One. 2013;8:e76972. doi: 10.1371/journal.pone.0076972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kay LE, Torchia DA, Bax A. Backbone dynamics of proteins as studied by 15N inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry. 1989;28:8972–8979. doi: 10.1021/bi00449a003. [DOI] [PubMed] [Google Scholar]
- 52.Farrow NA, Zhang O, Forman-Kay JD, Kay LE. Characterization of the backbone dynamics of folded and denatured states of an SH3 domain. Biochemistry. 1997;36:2390–2402. doi: 10.1021/bi962548h. [DOI] [PubMed] [Google Scholar]
- 53.Marsh JA, Singh VK, Jia Z, Forman-Kay JD. Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation. Protein Sci. 2006;15:2795–2804. doi: 10.1110/ps.062465306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roehrl MH, Wang JY, Wagner G. A general framework for development and data analysis of competitive high-throughput screens for small-molecule inhibitors of protein- protein interactions by fluorescence polarization. Biochemistry. 2004;43:16056–16066. doi: 10.1021/bi048233g. [DOI] [PubMed] [Google Scholar]
- 55.Mittag T, Orlicky S, Choy WY, Tang X, Lin H, Sicheri F, et al. Dynamic equilibrium engagement of a polyvalent ligand with a single-site receptor. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17772–17777. doi: 10.1073/pnas.0809222105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bozoky Z, Krzeminski M, Muhandiram R, Birtley JR, Al-Zahrani A, Thomas PJ, et al. Regulatory R region of the CFTR chloride channel is a dynamic integrator of phospho- dependent intra- and intermolecular interactions. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4427–4436. doi: 10.1073/pnas.1315104110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao H, Brautigam CA, Ghirlando R, Schuck P. Overview of current methods in sedimentation velocity and sedimentation equilibrium analytical ultracentrifugation. Curr Protoc Protein Sci. 2013;Chapter 20(Unit 20):12. doi: 10.1002/0471140864.ps2012s71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bohr C, Hasselbalch K, Krogh A. Ueber einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt. Skandinavisches Archiv Für Physiologie. 1904;16:402–412. [Google Scholar]
- 59.Changeux JP. The feedback control mechanisms of biosynthetic L-threonine deaminase by L-isoleucine. Cold Spring Harbor symposia on quantitative biology. 1961;26:313–318. doi: 10.1101/sqb.1961.026.01.037. [DOI] [PubMed] [Google Scholar]
- 60.Gerhart JC, Pardee AB. The enzymology of control by feedback inhibition. The Journal of biological chemistry. 1962;237:891–896. [PubMed] [Google Scholar]
- 61.Kamadurai HB, Qiu Y, Deng A, Harrison JS, Macdonald C, Actis M, et al. Mechanism of ubiquitin ligation and lysine prioritization by a HECT E3. eLife. 2013;2:e00828. doi: 10.7554/eLife.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott DC, Sviderskiy VO, Monda JK, Lydeard JR, Cho SE, Harper JW, et al. Structure of a RING E3 trapped in action reveals ligation mechanism for the ubiquitin- like protein NEDD8. Cell. 2014;157:1671–1684. doi: 10.1016/j.cell.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown NG, VanderLinden R, Watson ER, Qiao R, Grace CR, Yamaguchi M, et al. RING E3 mechanism for ubiquitin ligation to a disordered substrate visualized for human anaphase-promoting complex. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1504161112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol Cell. 2003;11:1445–1456. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 65.Wu H. Higher-order assemblies in a new paradigm of signal transduction. Cell. 2013;153:287–292. doi: 10.1016/j.cell.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen Ba AN, Yeh BJ, van Dyk D, Davidson AR, Andrews BJ, Weiss EL, et al. Proteome-wide discovery of evolutionary conserved sequences in disordered regions. Science signaling. 2012;5:rs1. doi: 10.1126/scisignal.2002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davey NE, Van Roey K, Weatheritt RJ, Toedt G, Uyar B, Altenberg B, et al. Attributes of short linear motifs. Molecular bioSystems. 2012;8:268–281. doi: 10.1039/c1mb05231d. [DOI] [PubMed] [Google Scholar]
- 68.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cancer Genome Atlas Research N. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130:127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 73.Wu K, Kovacev J, Pan ZQ. Priming and extending: a UbcH5/Cdc34 E2 handoff mechanism for polyubiquitination on a SCF substrate. Mol Cell. 2010;37:784–796. doi: 10.1016/j.molcel.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown NG, Watson ER, Weissmann F, Jarvis MA, VanderLinden R, Grace CR, et al. Mechanism of polyubiquitination by human anaphase-promoting complex: RING repurposing for ubiquitin chain assembly. Mol Cell. 2014;56:246–260. doi: 10.1016/j.molcel.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 76.Keller R. The computer aided resonance assignment tutorial. 1. Germany: 2004. [Google Scholar]
- 77.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown PH, Schuck P. Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys J. 2006;90:4651–4661. doi: 10.1529/biophysj.106.081372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 80.Huang DT, Zhuang M, Ayrault O, Schulman BA. Identification of conjugation specificity determinants unmasks vestigial preference for ubiquitin within the NEDD8 E2. Nature structural & molecular biology. 2008;15:280–287. doi: 10.1038/nsmb.1387. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, Li Y, Wu Z, Wang H, Tan H, Peng J. JUMP: a tag-based database search tool for peptide identification with high sensitivity and accuracy. Mol Cell Proteomics. 2014;13:3663–3673. doi: 10.1074/mcp.O114.039586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.