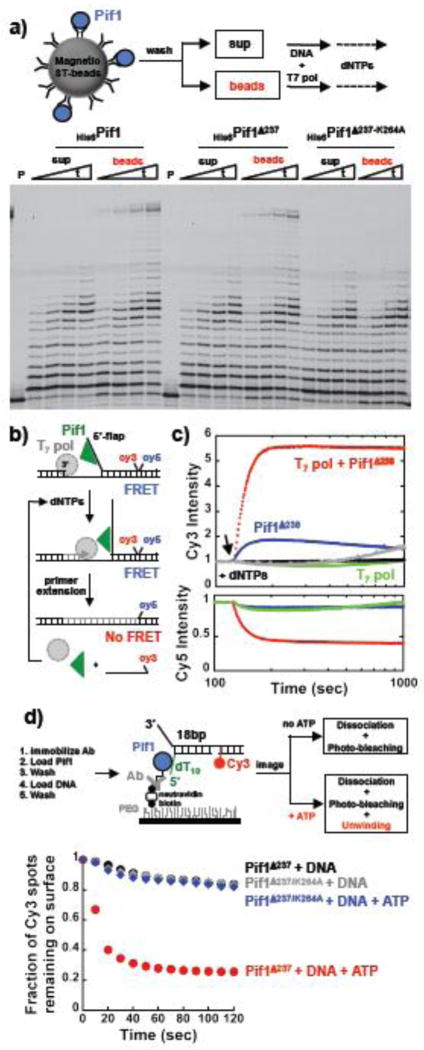
Figure 2. A monomer of Pif1 is sufficient to stimulate T7-DNA polymerase activity by unwinding the downstream dsDNA. a).
Primer extension reactions performed with Pif1 immobilized to streptavidin bead via a biotinylated penta-His antibody. b) Schematic of the FRET-based unwinding assays. c) FRET-based assays using 20 nM DNA and monitoring the strand displacement DNA synthesis/unwinding by 20 nM T7-Pol (green) or 20 nM T7-Pol and 15 nM Pif1238–859 (red) or 15 nM Pif1238–859 (blue). The same experiments for the ATPase inactive Pif1 are shown in black and gray. The arrow indicates the time of addition of 100 μM dNTPs. d) Schematic of single molecule approach to detect helicase activity of Pif1 monomers immobilized on a surface. The graph shows the fraction of Cy3 spots remaining on the surface after addition of either image buffer or image buffer + ATP. Pif1238–859 or its ATPase inactive variant K264A were used in these experiments.