Fig. 3.
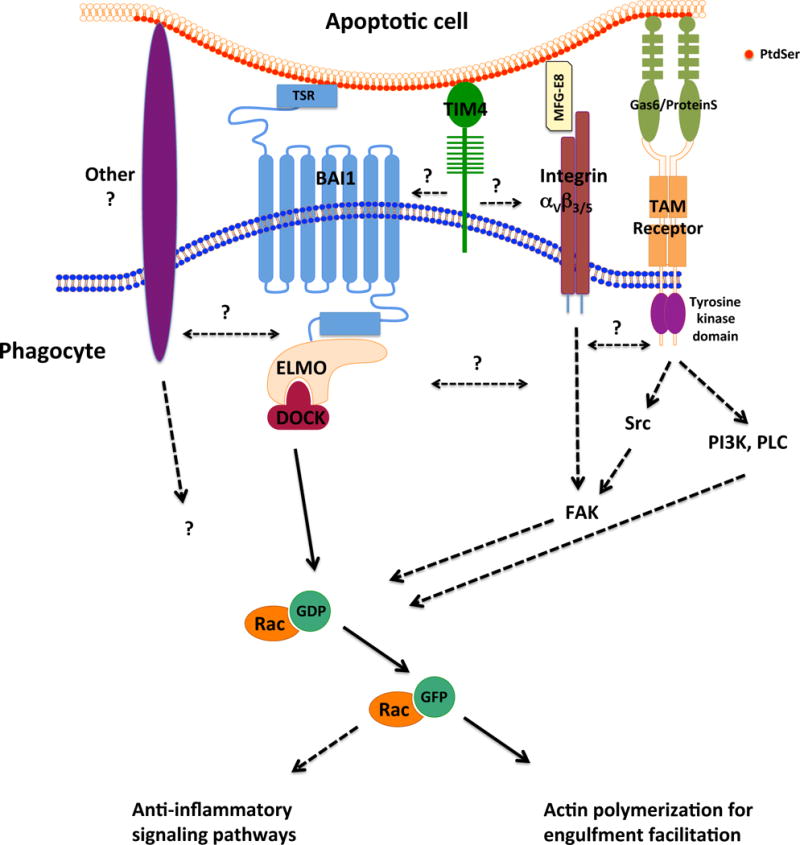
Signaling pathways elicited by three PtdSer recognition receptors. Binding of the apoptotic cell to the phagocyte triggers signaling pathways. BAI1 is a 7-transmembrane receptor that directly binds the PtdSer on the surface of an apoptotic cell, resulting in the recruitment of the Engulfment and cell motility (ELMO)/Downstream of Crk (DOCK) complex, which functions as a guanine exchange factor for the small GTPase Rac39. Rac activation promotes actin cytoskeleton remodeling required for the engulfment of the apoptotic corpse. Integrins αVβ3 or αVβ5 and the Tyro Axl Mer (TAM) family receptors bind apoptotic cells indirectly, via PtdSer-bound bridging molecules MFG-E8, Gas6 or ProteinS, resulting in the activation of the focal adhesion kinase (FAK) and contributing to the activation of Rac114. TAM receptors are tyrosine kinases that also activate cell signaling pathways involving the kinases Src and phosphatidylinositol-3-kinase (PI3K) and phospholipase C (PLC)114, 115. TIM4 functions as a tethering receptor bringing the apoptotic cell in contact with signaling engulfment receptors, and signal through co-receptors. The extent of the connection between the signals elicited by different engulfment receptors awaits further characterization.
