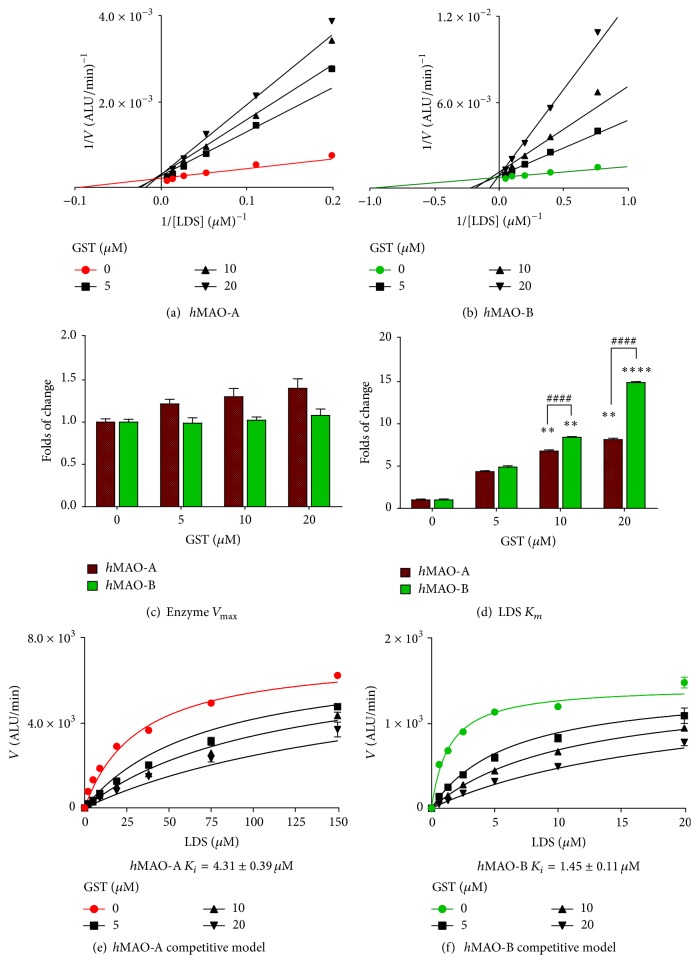
Figure 3.
Mode of inhibition of recombinant human monoamine oxidases (hMAO-A and hMAO-B) by genistein (GST) compared to control the initial velocity (V). Lineweaver-Burk plots for hMAO-A (a) and hMAO-B (b) with a gradual increase of luciferin derivative substrate (LDS). Linear regression data are presented as mean ± SEM of n = 3. The maximum velocity (V max ± SEM) (c) and Michaelis constant (K m ± SEM) of LDS (d) parameters folds of change were measured with a gradual increase of GST concentrations in both isozymes. GST inhibitor constant (K i) was determined using the competitive inhibition model for hMAO-A (e) and hMAO-B (f) as the best fit. Regression data are presented as the mean ± SEM of n = 3. The significance of difference between the controls and treatments was determined using one-way ANOVA followed by Dunnett's multiple comparisons test and between two groups of each concentration using t-test. ∗∗ p < 0.01; ∗∗∗∗ or #### p < 0.0001.