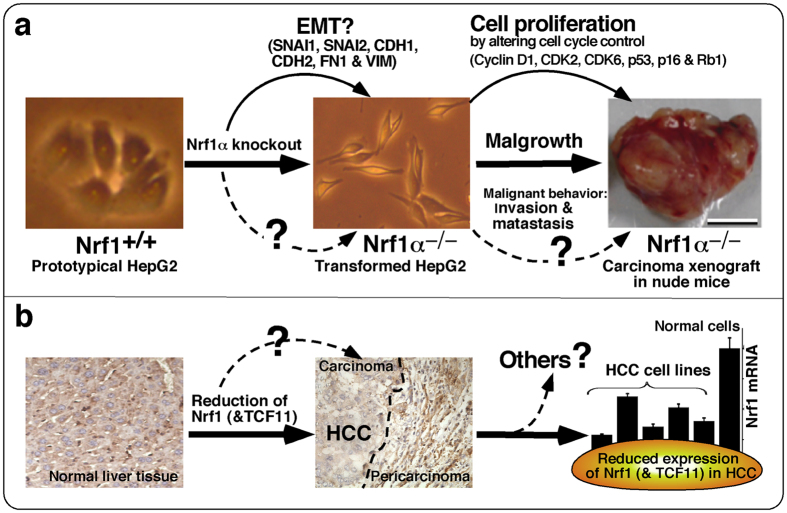
Figure 15. Schematic modeling of Nrf1α−/−-promoted EMT and other cell processes in the liver cancer development.
(a) Schematic representation of Nrf1α−/−-promoted EMT and other cell processes involved in the mouse xenograft model. The wild-type (i.e. Nrf1α+/+) epithelial-like HepG2 cells are subjected to TALENs-directed site-specific knockout of Nrf1α in order to yield the resultant bi-allelic mutant (i.e. Nrf1α−/−) cells that are endowed with the mesenchymal-like characteristics. The phenotypic change is regarded as the epithelial-mesenchymal transient (EMT, which occurs at the primary tumour foci), whilst EMT is reversible to the process being referred to as the mesenchymal-epithelial transient (MET, which takes place at suitable metastatic sites). The putative EMT dedifferentiation is promoted to acquire the high-metastatic potentials following Nrf1α-specific knockout, as is accompanied by enhanced expression of Nrf1β, in the hepatocellular carcinoma HepG2 cells and relevant xenograft model mice. (b) The human constitutive expression of Nrf1α, but not of Nrf1β, is markedly attenuated or even abolished in the low-differentiated high-metastatic hepatocellular carcinoma, amongst a series of paired carcinomas nodules and surrounding (pericarcinoma or para-carcinoma) tissues. The hepatic Nrf1α expression is further deteriorated by chronic (uncontrollable) inflammatory, fibrotic and cirrhotic lesions. Collectively, our evidence that has been presented (and some data not shown) in the paper, together with previous studies revealing that potential switches between EMT and MET control the hepatocarcinogenesis and its malignant progression (i.e. transformation, malgrowth, invasion and metastasis), has let us to propose that Nrf1α (but not Nrf1β) might monitor such EMT-MET switches to repress hepatic cancer development. The notion is also supported by the fact that the putative functional loss of Nrf1α (though Nrf1β/γ are also lost) in the mouse liver results in the hepatic spontaneous cancer33,34. Overall, Nrf1α is of significant importance in the physio-pathological origin and development, but further studies are warranted to address a new open question of whether (and how) Nrf1α monitors the putative EMT-MET reprogramming during the embryonic and cancer development.