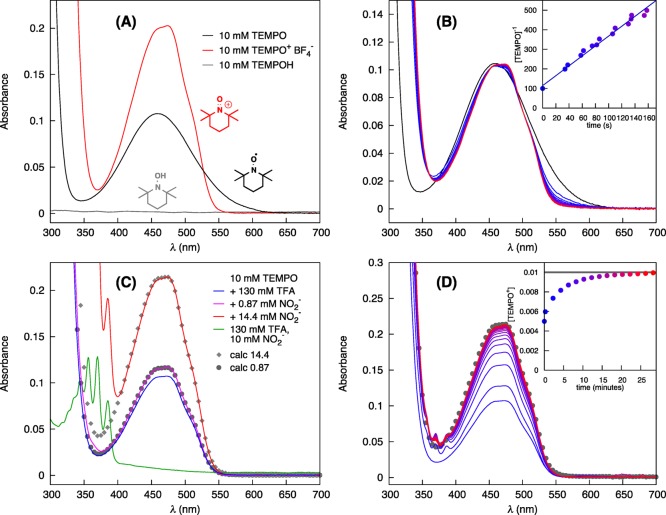
Figure 1.
UV–visible studies of TEMPO disproportionation and reactivity with NaNO2 under acidic conditions in acetonitrile. (A) Spectra of 10 mM TEMPO+, TEMPO, and TEMPOH in CH3CN.23 (B) Spectra obtained following addition of trifluoroacetic acid (TFAH) to a 10 mM solution of TEMPO, corresponding to TEMPO disproportionation into TEMPO+ and TEMPOH eq 6. The linear fit to [TEMPO]−1 (inset) incorporates data from three independent experiments. Conditions: 10 mM TEMPO in CH3CN, 130 mM TFA added at t = 0. (C) Spectral changes observed upon addition of NaNO2 (0.09 and 1.4 equiv) to a disproportionated-TEMPO solution in CH3CN/TFAH under N2. The changes reflect oxidation of TEMPOH to TEMPO+ by nitrite. The gray points represent the expected spectrum for full conversion of NO2– to NO or TEMPO to TEMPO+ depending on the limiting reagent. Conditions: 10 mM TEMPO in CH3CN with 130 mM TFA, 0.9 or 14.4 mM NaNO2, N2 atmosphere. (D) Aerobic oxidation of disproportionated TEMPO catalyzed by nitrite. The initial spectrum of TEMPO+ with colorless TEMPOH shifts to higher absorbance as more TEMPO+ is formed (blue → red, 2 min scan interval). The gray dotted spectrum depicts the spectrum expected if all TEMPO-based species are converted to TEMPO+. Conditions: 10 mM TEMPO in CH3CN with 130 mM TFA, 0.9 mM NaNO2 added at t = 0, 1 atm O2.