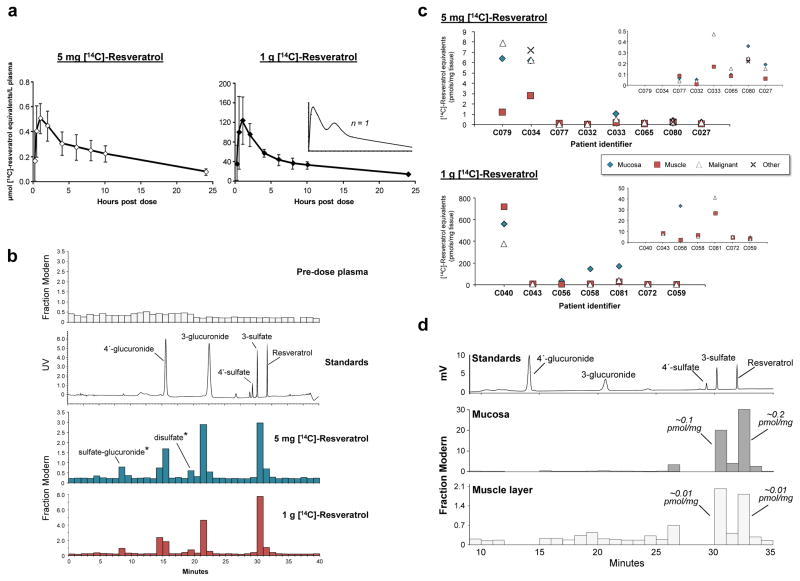
Figure 1. Comparison of the plasma pharmacokinetics and target tissue distribution of [14C]-resveratrol and its metabolites in humans following a low dietary achievable dose or high pharmacological dose.
(a–b) Healthy volunteers received a single [14C]-labelled oral dose of either 5 mg or 1 g resveratrol (44.5 kBq, 0.962 μSv) and plasma samples were taken over 24 h for determination of total [14C]-resveratrol equivalents by AMS analysis. (a) Graphs show average (± SD) concentrations for 10 volunteers per group, whilst the inset represents a single participant to illustrate the second peak maxima commonly observed with resveratrol due to enterohepatic recirculation. (b) Plasma metabolite profiles determined by HPLC-AMS analysis of selected samples from one patient on each resveratrol dose, taken 1 h after ingestion. Also included are a pre-dose plasma sample for determination of background levels of radiocarbon and a UV chromatogram from the analysis of authentic metabolite standards. Peaks designated by * were tentatively assigned on the basis of their chromatographic properties, since synthetic standards were not available. (c) Levels of [14C]-resveratrol equivalents in tissues of patients with colorectal cancer that received either 5 mg (n=8) or 1 g (n=7) resveratrol daily for 1 week prior to surgery, with the last dose being [14C]-radiolabelled. Where possible, malignant tissue and normal colorectal mucosa and muscle were obtained for each patient. For some participants, other tissue types (fat, ovarian tumour) were also available for analysis (Supplementary Table 2). One patient in the high dose group had surgery delayed by 6 days after taking [14C]-resveratrol and has been excluded. Enlargements are included as insets to enable comparisons at lower concentrations. (d) Metabolite profile in colorectal mucosa and muscle tissue of a patient that received 5 mg [14C]-resveratrol, determined by HPLC-AMS analysis. Peaks of radiocarbon in both tissue types correspond to resveratrol and its 3-sulfate, based on similarity of retention times to authentic standards, and the concentrations stated translate to μM, assuming 1g of tissue equates to 1mL.