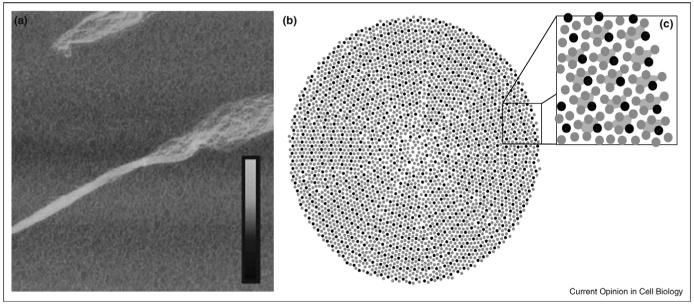
Figure 1.
Supramolecular organization of collagen fibrils. (a) The superhelical twist of individual fibrillar elements can be seen in this atomic force microscopy image of a mechanically disrupted collagen fibril (ref. [51], reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. Copyright Wiley-Liss, 2006). The box size is 5 μm × 5 μm and the inset height scale corresponds to 0-30 nm. (b-c) Cross-section model of molecular packing in collagen fibrils (adapted with permission from ref. [19]. Copyright Elsevier, 2002). Thousands of individual collagen triple-helices interact to form a single fibril with both ordered and disordered packing features. Collagen microfibrils are formed by five collagen molecules in a staggered arrangement, shown connected by trapezoids in (c).