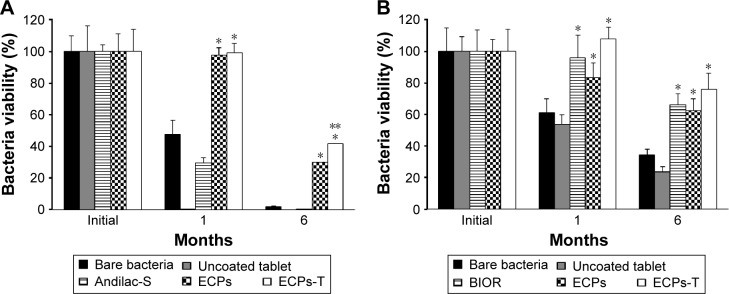
Figure 6.
Storage stability of (A) L. acidophilus and (B) E. faecalis in ECPs-T in comparison to corresponding bare bacteria, uncoated probiotic tablet, marketed products, and ECPs under ambient storage conditions (25°C/60% relative humidity) for 6 months.
Notes: To compare the bacterial storage viability, two marketed products, Andilac-S capsule (Il-Yang Pharm. Co., Ltd., Gyeonggi-do, Korea) or BIOR tablet (Unimed Pharmaceutical Inc., Seoul, Korea) containing L. acidophilus or E. faecalis, respectively, were used. Data represent the mean ± SD (n=3), and statistical analysis was performed using Student’s t-test; *P<0.05 versus bare bacteria; **P<0.05 versus ECPs.
Abbreviations: ECPs, enteric-coated pellets; ECPs-T, enteric-coated pellets embedded tablet; E. faecalis, Enterococcus faecalis; L. acidophilus, Lactobacillus acidophilus; SD, standard deviation.