Abstract
AIM
Gastric disorders affect the gastric slow wave. The cutaneous electrogastrogram (EGG) evaluates the electrical potential of the slow wave but is limited by the volume conduction properties of the abdominal wall. The magnetogastrogram (MGG) evaluates the gastric magnetic field activity and is not affected as much by the volume conductor properties of the abdominal wall. We hypothesized that MGG would not be as sensitive to body mass index as EGG.
METHODS
We simultaneously recorded gastric slow wave signals with mucosal electrodes, a Superconducting Quantum Interference Device magnetometer (SQUID) and cutaneous electrodes before and after a test meal. Data were recorded from representative pools of human volunteers. The sensitivity of EGG and MGG was compared to the body mass index and waist circumference of volunteers.
RESULTS
The study population had good linear regression of their Waist circumference (Wc) and Body Mass Index (BMI) (regression coefficient, R=0.9). The mean BMI of the study population was 29.2 ±1.8 kgm−2 and mean Wc 35.7±1.4 inch. We found that while subjects with BMI≥25 showed significant reduction in post-prandial EGG sensitivity, only subjects with BMI≥30 showed similar reduction in post-prandial MGG sensitivity. Sensitivity of SOBI “EGG and MGG” was not affected by the anthropometric measurements.
CONCLUSIONS
Compared to electrogastrogram, the sensitivity of the magnetogastrogram is less affected by changes in body mass index and waist circumference. The use of Second Order Blind Identification (SOBI) increased the sensitivity of EGG and MGG recordings and was not affected by BMI or waist circumference.
Keywords: Magnetogastrogram, Electrogastrogam, Gastric mucosal myoelectrical signal, Body mass index, Waist circumference
INTRODUCTION
Propagating electrical waves in the stomach, known as slow waves, coordinate the mixing and propulsion of foods. The slow wave electrical activity in the stomach musculature produces both extracellular electric potential and magnetic fields. EGG, which uses multiple electrodes placed on the abdomen to record cutaneous electric potential[1] and MGG that utilizes the highly sensitive superconducting quantum interference device (SQUID) to record corresponding magnetic field potential[2]; both provide noninvasive methods for recording of electric or magnetic fields resulting from gastric slow wave activity. EGGs have received limited clinical acceptance while MGGs are still used only for research purposes. This is in part due to a lack of detailed knowledge about the underlying slow wave activity and other limitations related to technical and physiological recording of EGGs and MGGs[3,4].
It is known that EGG signals are relatively weak and difficult to record reliably while magnetic fields are theoretically less attenuated by the low-conductivity fat layers present in the body and thus may provide a significant advantage over EGGs[5,6]. The alternation in conductivity of abdominal layers between low and high values distorts and attenuates the electrical potential of the gastric slow wave such that EGG represents the smoothed and summed contribution of a continuum of slow wave sources[6,7]. In theory, abdominal fat is an effective electrical insulator with low electrical conductivity that significantly reduces the amplitude of cutaneous EGG electric potential[8]. Magnetic fields however, are mediated by permeability instead of conductivity, and since the permeability of fat and other tissues are nearly equal to that of free space; we expect the effect on MGG conductivity to be minimal. Model studies have consistently demonstrated that these conductivity properties of abdominal layers affect EGG more than MGG[5,6,9].
In this study, we will examine the effect of BMI and waist circumference on the sensitivity of EGG and MGG in detecting simultaneously recorded internal gastric slow waveforms and corresponding dominant frequency during fasted and fed states in human subjects. Our hypothesis is that MGG sensitivity will be less affected by anthropometric measurements.
MATERIALS
Human Subjects
Twenty-eight normal human volunteers [17 men and 11 women, aged 19 to 45 years, BMI range 18 to 61.3 (12 normal, 8 overweight, 8 obese) and range of waist circumference from 27.5 to 53.5 inches] participated in this study. None of the volunteers had prior history of gastrointestinal disease or surgery and none was on medications known to alter gastrointestinal motor or electrical activity. A pregnancy test was performed in all female volunteers prior to enrollment.
Experiment Overview
We simultaneously measured multichannel MGG, EGG and gastric mucosal myoelectrical (EMG) signals on volunteers who came to the Vanderbilt General Clinical Research Center, after an overnight fast. The volunteers were selected to populate representative pools of various BMI categories. Mucosal electrode recording was the standard gastric slow wave signal from which we determined the sensitivity of simultaneously recorded MGG and EGG. All studies were reviewed and approved by Vanderbilt’s Committee for protection of Human Subjects. We placed the naso-gastric (NG), mucosal suction EMG tube in the pyloro-antral region; along the greater curvature of the stomach and verified appropriate placement of the NG tube electrodes in the gastric antrum, using plain x-ray as seen in figure 1. Cutaneous electrodes and a SQUID magnetometer (Model 637, Tristan Technologies, San Diego, CA) recorded EGG and MGG signals respectively. Cutaneous electrodes consisted of four rows of four monopolar electrode pairs with a central reference placed on the abdomen above the stomach along the longitudinal axis. Volunteers were then placed underneath the SQUID magnetometer in a magnetically shielded room (Amuneal, Philadelphia, PA). The subject’s abdomen was positioned such that the highest point of the abdomen while supine was in close approximation with the bottom of the SQUID dewar but not touching it as the subject inspired maximally. Simultaneous mucosal EMG, EGG and MGG data were recorded during fasting for a period of 30 min. The volunteers then ate a standardized 300 kcal turkey sandwich meal with a clear liquid (water). After the meal, we recorded the postprandial signal for a period of 1 h. At several times during data collection, the subjects were asked to suspend respiration to allow comparison of noise reduction techniques.
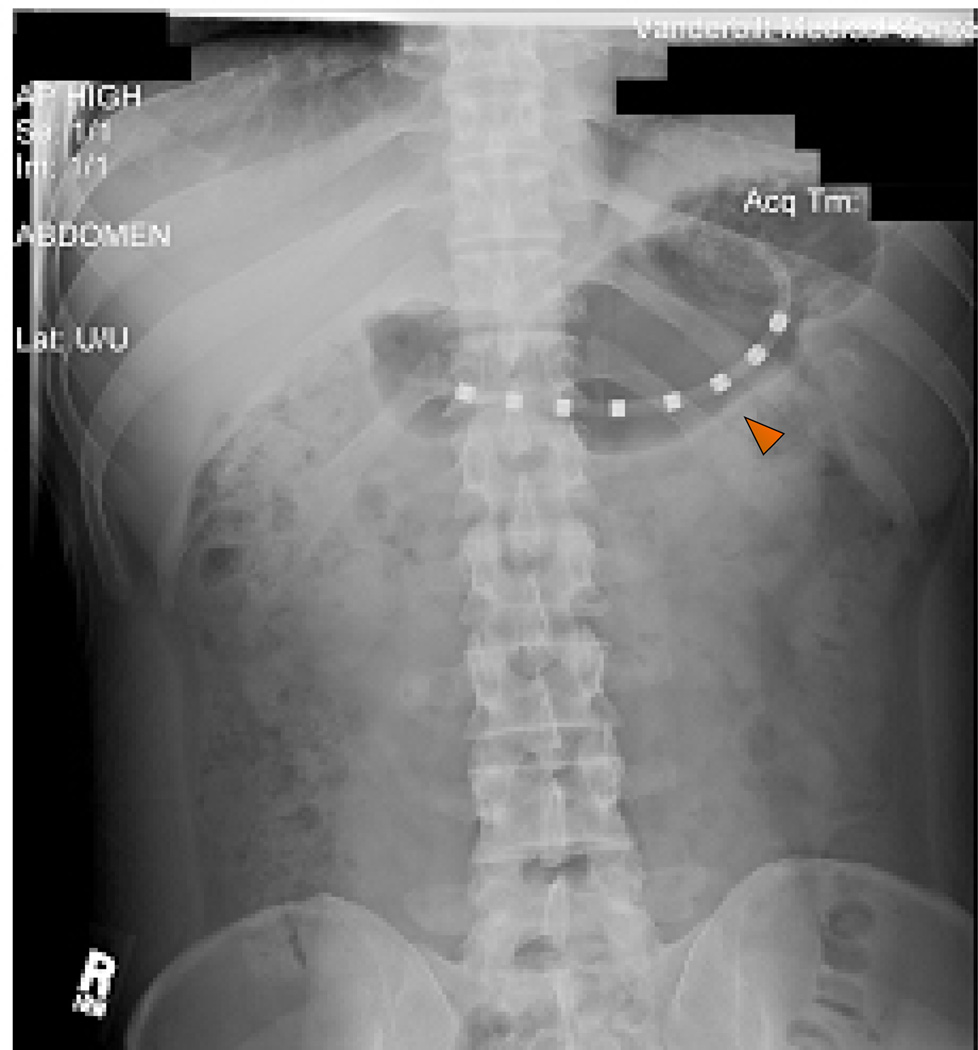
Figure 1.
Plain abdominal radiograph. Red arrow in the plain abdominal film shows gastric mucosal electrode on the antrum of the stomach for EMG recording.
Anthropometric Measurements: Subject mass was measured on the same digital scale (Seca, Hamburg, Germany) to the nearest 0.1 kg. Height was measured with a wall-mounted stadiometer (Perspective Enterprises, Portage, MI). Waist circumference (Wc) was measured with the umbilicus as a reference point.
Mucosal Electrode
Our mucosal suction electrode is a modification of the electrode of Monges and Salducci[10]. It consists of eight platinum EMG electrode rings (Dentsleeve International, USA) located at the tip of a custom-fabricated flexible silicon catheter. The EMG rings connect to extension adaptors and to an electrode amplifier (Biosemi, Amsterdam, The Netherlands). The electrodes are placed 1.5 cm apart. Alternate spaces between electrodes have a 0.1 cm fenestration at the midpoint that connects through a central lumen to an outlet and suction device. Pressure up to −100 mm Hg was applied to achieve close electrode-mucosal contact during pre-prandial signal recording. We relied on increased intragastric pressure for similar contact during the postprandial study.
Cutaneous Electrode
EGG electrodes were pre-gelled, disposable, stick-on electrodes from Rochester Electro-medical Inc.
SQUID Magnetometer Measurements
The SQUID converts magnetic flux incident on detection coils from 37 sensors at different locations and orientations into voltage signals. A set of 19 detection coils oriented normal to the body habitus are arranged in gradiometer format spaced in a hexagonal close-packed array in the x–y plane over an area with a 10 cm radius. Ten additional coils record tangential components of biological magnetic signals and eight more provide a reference recording of ambient magnetic noise.
Data acquisition
Electrode signals (EGG and EMG) were acquired at 256 Hz with the electrode amplifier and resampled to 30 Hz. SQUID signals were passed into a preamplifier stage (Model 5000, Quantum Design, San Diego) with a gain of 5 and a low-pass filter set to 1 kHz. Data were acquired at 3 kHz and subsequently down-sampled to 30 Hz.
Signal processing
To investigate the effect of BMI on multichannel MGG and EGG in normal human subjects, all simultaneously recorded mucosal, cutaneous and SQUID signals were subjected to spectral analysis. Recorded signals were loaded into MATLAB™ (Mathworks, Natick, MA, USA). All frequency spectra were computed with fast Fourier transform (FFT). For this study, we first investigated the effect of BMI and Wc on the sensitivity of simultaneously-recorded, unfiltered MGG and EGG waveform and corresponding dominant frequency, determined from simultaneously recorded EMG signal.
Next, we applied the tri-step signal processing algorithm described elsewhere by this group[11,12] that minimizes the interfering noise signals. All signals were first filtered using a second-order Butterworth filter with a bandpass of 1–60 cycles per minute (cpm). Furthermore, we applied the second-order blind identification (SOBI) signal processing and subsequently, identified the resulting source components and their corresponding dominant frequencies. A detailed description of SOBI and its mathematical formulations are available elsewhere[11–13]. Sensitivity data were also computed as above for the SOBI derived surface current signals of MGG and EGG. These sensitivity data were also compared to the anthropometric measurements.
Statistical analysis
A binary classification sensitivity test of MGG and EGG detection of simultaneously recorded EMG signals shown in figure 2 and 3 during pre and post-prandial segments of the study was computed using the 2 by 2 table below (Table 1). We analyzed 120-s-long data segments in steps of 20 s (e.g., 0–120 s, 20–140 s, etc). Signal is present at a specific time point when a gastric type signal is identified by EMG. “Signal Detected” refers to a similar signal identified by MGG or EGG. Gastric signal was defined as signal with sinusoidal waveforms and a single dominant peak frequency in the range of 2.5 to 4.0 cpm[11,12]. We classified parametric distributions into Gaussian or non-Gaussian and applied unpaired Student’s t-test for comparison of data using Excel 2010 (p<0.05 for significance). Linear regression was used to determine the correlation between variables. Sensitivity data of the pre and postprandial study were compared to the BMI and waist circumference of volunteers in scatter plot. All mean data are reported±standard error of mean (SEM) and all sensitivity data are reported as percentages.
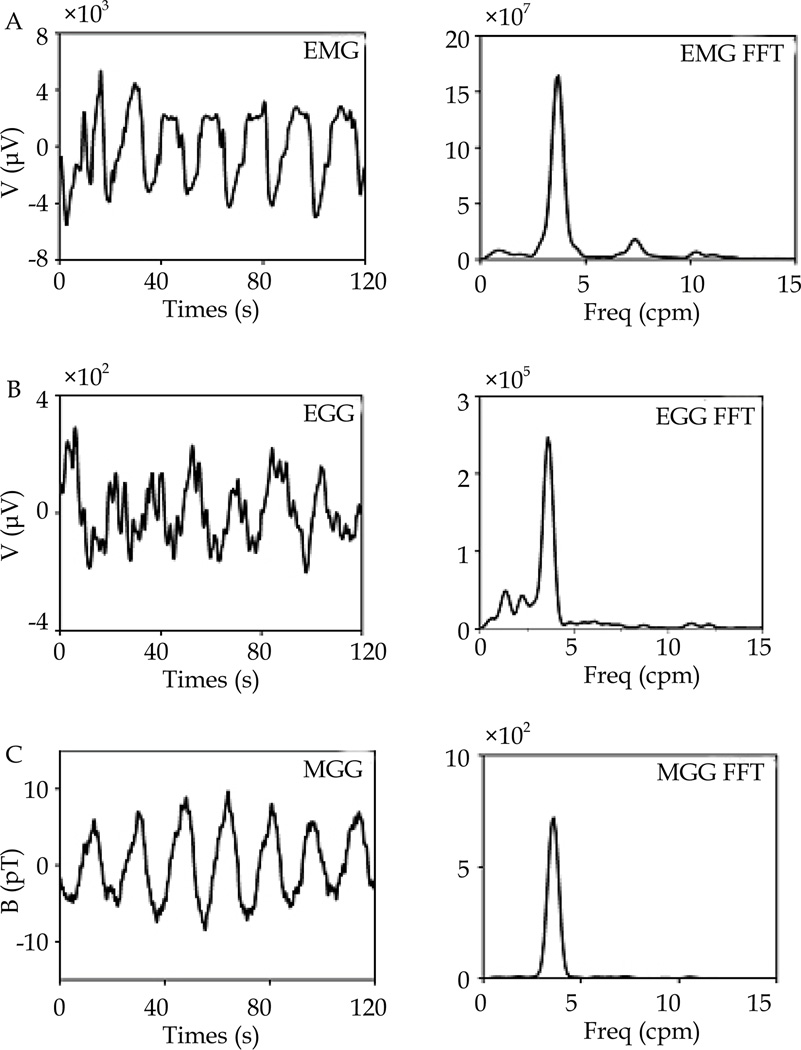
Figure 2.
A, B, C: 120 sec time segment of simultaneously recorded EMG, EGG and MGG signals and corresponding FFT dominant frequency, from a volunteers in this study. Signals analyzed in this study. MGG (picotesla unit), EMG and EGG (microvolt units).
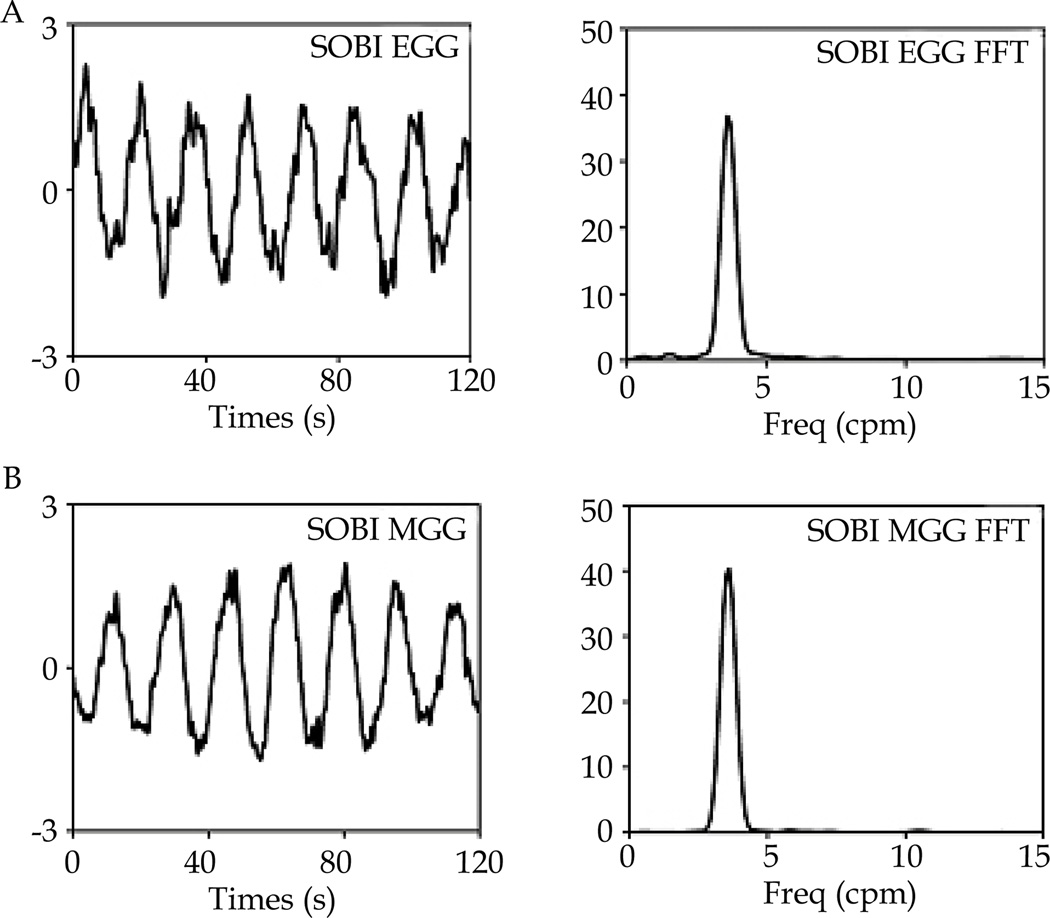
Figure 3.
A, B: SOBI EGG and SOBI MGG signals with corresponding FFT dominant frequency. Signals were isolated from analysis of above signals depicted in figure 2.
Table 1.
Shows the 2 by 2 table.
| Signal Detected | Signal Not Detected | |
|---|---|---|
| Signal Present | True positive | False negative |
| Signal Absent | False positive | True negative |
Sensitivity=true positive/true positive+false negative.
RESULTS
Recordings of simultaneous pre and postprandial signals from the gastric mucosal EMG, multichannel SQUID MGG and the cutaneous EGG were made in 19 of 25 normal human volunteers who successfully completed this study. Simultaneous signals were recorded in 5 volunteers with normal BMI, and 7 volunteers in overweight and obese groups respectively. Difficulty in achieving contact between the mucosal EMG electrodes and the gastric mucosa largely accounted for this discrepancy. An example of postprandial data obtained from one of the studies is shown in figures 2 and 3. The figure shows the unfiltered EMG, EGG and MGG signals and their SOBI-derived components, all with their corresponding FFTs. This figure was generated from simultaneously recorded signals in one of the volunteers. The regular gastric slow wave activity with a frequency of approximately 3 cpm is clearly evident in all modalities. The study population had good linear regression of their Wc and BMI (regression coefficient, R=0.9). The mean BMI of the study population was 29.2± 1.8 kgm−2 and mean Wc 35.7±1.4 inch. Anthropometric measurements from the different BMI groups are shown in table 2.
Table 2.
Shows anthropometric measurements, mean percentage of gastric slow waves analyzed and pre-prandial gastric slow wave detection sensitivity data in the study subpopulations.
| BMI (kgm−2) | Wc (inch) | % of GSW. | Pre-prandial sensitivity | ||||
|---|---|---|---|---|---|---|---|
| MGG | SOBI-MGG | EGG | SOBI-EGG | ||||
| Normal | 21.8 ± 1.1 | 32.1 ± 1.7 | 5.7±1.2 | 58.4 ± 11.5 | 94.6 ± 3.3 | 77.1± 12 | 100.0 ± 0.0 |
| Over-weight | 27.8 ± 0.5 | 36.2 ± 0.5 | 5.1±1.2 | 44.8 ± 10.2 | 97.1 ± 1.7 | 38.3 ± 9.7 | 98.3 ± 1.7 |
| Obese | 40.2 ± 3.8 | 42.6 ± 2.9 | 4.3±1.4 | 32.6 ± 10.7 | 96.6 ± 2.2 | 30.6 ±9.5 | 100.0 ± 0.0 |
For convenience of description, we will apply the following notations: MGG and EGG refers to raw magnetogastrogram and electrogastrogram signal with recognizable waveform and dominant frequency consistent with our stated criteria and without Butterworth digital filter or SOBI algorithm applied. SOBI-MGG and SOBI-EGG refer to the same signals processed using the SOBI algorithm to identify gastric signal components.
Pre-prandial time segments of normal gastric slow wave signals (N=872) and similar time segment signals from postprandial recordings (N=1948) were identified and analyzed in this study. There was no statistical difference in the percentage of normal gastric slow wave signals analyzed in the different BMI subpopulations (p≥0.5). Table 2 and 3 show the mean percentage of the slow wave signals analyzed in the different subpopulations. In all analyzed time segments of recorded slow wave signals, the SOBI algorithm identified gastric slow wave type signals otherwise called, SOBI-MGG and EGG; from signals with obvious 3cpm sinusoidal waveforms and also from other signals without similar recognizable configuration but were simultaneously recorded with mucosal EMG electrode signals. Most of the unrecognizable magnetic and electrical signals were contaminated by biologic and non-biological noise components that were most often amenable to separation by the application of SOBI algorithm to identify SOBI- MGG and EGG. We found no change in pre to postprandial slow wave dominant frequency in any modality of gastric slow wave measurement (p≥0.06). The mean EMG dominant frequency was 2.99±0.03 cpm pre-prandial and 3.00±0.01 cpm postprandial. MGG and EGG also had similar dominant frequency in pre and post-prandial periods; 3.00±0.03 cpm pre-prandial and 2.96±0.01 cpm postprandial respectively. A linear regression model determined good correlation of dominant frequencies of MGG and EGG with those of simultaneously detected EMG signals in both pre and post-prandial periods (R=0.9).
Table 3.
Shows anthropometric measurements, mean percentage of gastric slow waves analyzed and post-prandial gastric slow wave detection sensitivity data in the study subpopulations.
| BMI (kgm−2) | Wc (inch) | % of GSW. | Post-prandial sensitivity | ||||
|---|---|---|---|---|---|---|---|
| MGG | SOBI-MGG | EGG | SOBI-EGG | ||||
| Normal | 21.8 ± 1.1 | 32.1 ± 1.7 | 4.5±1.2 | 84.4 ± 6.5 | 99.3 ± 0.4 | 92.6 ± 4.5 | 100.0 ± 0.0 |
| Over-weight | 27.8 ± 0.5 | 36.2 ± 0.5 | 5.0±0.9 | 80.7 ± 6.0 | 100.0±0.0 | 59.0 ± 8.9 | 98.6 ± 0.7 |
| Obese | 40.2 ± 3.8 | 42.6 ± 2.9 | 4.7±0.9 | 56.8 ± 7.2 | 96.0 ± 1.7 | 53.4 ± 6.7 | 100.0 ± 0.0 |
In this study, we observed an increase in EGG sensitivity from 45.7±7.1 to 65.8±5.6 in pre-prandial compared to postprandial periods (p=0.03). A similar postprandial increase in MGG power was also observed. MGG sensitivity increased from 44.0±6.4 in pre-prandial study to 72.8±4.7 in postprandial period (p=0.0008).
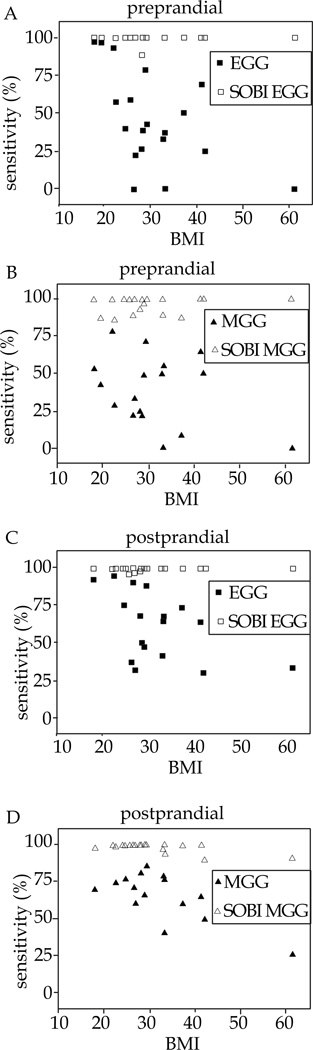
The relatively higher SEM from pre-prandial sensitivity data in table 2 compared to post-prandial data in table 3 is consistent with increased variation in EGG and MGG sensitivity data recorded when the subjects were fasted. Figure 4 shows scatterplots that demonstrate a progressive increase in BMI resulting in a decline in sensitivity of both MGG and EGG during both fasted and fed states. However, a more pronounced distinction emerged when we evaluated the effect of BMI on the sensitivity of these noninvasive gastric slow wave detection methods in the various subpopulations. We observed a significant reduction in EGG sensitivity in obese and overweight populations compared to normal BMI subjects in both pre and post prandial studies (p≤0.01) but found no difference in the data when overweight and obese subjects were compared with same t-test (p≥0.6). MGG sensitivity on the other hand, presented an entirely different picture. A significant reduction in post-prandial MGG sensitivity was observed in only the obese subpopulation compared to normal and overweight (p=0.02). Also, there was no statistical difference in pre-prandial MGG sensitivity data from all BMI subpopulation (p≥0.1). In other words, our data suggests that while subjects with BMI of 25 and greater showed significant reduction in post-prandial EGG sensitivity, only subjects with BMI of 30 and greater showed similar reduction in post-prandial MGG sensitivity. These changes paralleled increased waist circumference in these BMI populations but was not demonstrated in a similar comparison of SOBI EGG and MGG sensitivity with anthropometric measurements in these subpopulations. Figure 4 shows a progressive decline of MGG and EGG sensitivity with increasing BMI in our study population, but sensitivity of SOBI MGG and EGG is not affected by increasing BMI and Wc in either pre or post prandial studies.
Figure 4.
Shows the effect of BMI on sensitivity of MGG and EGG for detection of simultaneous GEA signal with EMG. EGG and MGG sensitivity diminishes with increasing BMI in both pre-prandial and post-prandial study but sensitivity of SOBI component is not affected by BMI.
DISCUSSION
Reports have noted the presence of abnormal slow wave propagation patterns and signal characteristics associated with several gastric disorders, namely gastric outlet obstruction[14], gastroparesis[15], gastric myoelectrical dysrhythmia[16], gastric atrophy and hypertrophy[17], diabetic gastropathy[18] and Chagas disease[19]. Concerns about the diagnostic proficiency of EGG and MGG signals have incentivized increased research aimed at improving our knowledge of the characteristics of these signals and the factors that affect them, as well as ways and means to improve the signal quality and diagnostic information. Of the spatial parameters that describe slow wave activity, namely: amplitude, frequency, phase, propagation velocity that are known to change in disease states; it has been suggested that the slow wave frequency of EGG is the only reliable information obtainable from cutaneous recordings[20,21]. In contrast however, MGG which also measures the same underlying electrical activity as EGG, are not affected by conductivity properties of volume conductors but it is affected by the distance from the source signal[5,6,22–24]. Other studies have demonstrated good correlation of MGG with internally placed serosal electrodes in identification of slow wave frequency gradient and propagation characteristics[25,26]. Consequently, there is growing interest among researchers to determine the influence of body mass index (BMI) on the sensitivity of MGG measurements, since this has never been established. Several reports have described the use of anthropometric measurements like waist circumference and BMI to predict visceral adiposity[27,28]. Obviously, the challenge for non-invasive surface potential measurements with increased BMI and waist circumference appears to be a combination of conductivity properties of all structures between the signal source (i.e. the stomach) and signal detectors, as well as the thickness or geometric distance[5,6,22,24]. The effect of other impediments like biologic and non-biologic noise components, on detection of these surface potentials has also been described[11].
Studies from our group have used surface current density (SCD) methods to localize the underlying gastric slow wave signal sources and calculate the propagation direction and velocities from magnetic field signals[2,29]. Ongoing experimental investigations are looking at the effects of body habitus on the relationship of the amplitude and propagation velocity of EGG and MGG when compared to internal slow wave measurements. Alongside these studies designed to characterize the slow wave components, we have reported, novel methods for identifying slow wave frequencies from noninvasive biomagnetic and electrical measurements using a tri- step algorithm that incorporates a digital Butterworth filter for pre-processing of recorded measurements to remove baseline drift and high frequency noise, and applies second-order blind identification (SOBI) to separate and identify signal source components[11].
The effect of respiration and other noise contaminants on magnetic field and electrical potential has been reported along with methods utilized to minimize or eliminate such contaminants[8,11,12]. Intermittent breath holds for 1 minute duration several times during signal recording served as a noise reduction technique in this study. However, signal processing with digital filters and SOBI to identify the specific signal of interest and eliminate other contaminants has been simplified by the algorithm described in this report. SOBI is a blind-source separation technique[11–13] that exploits the second-order statistics of the measurements to compute an estimate of the mixing matrix. The key step in the SOBI algorithm is to rotate a set of time-lagged cross-correlation matrices of SQUID or electrode data; such that they are jointly and approximately diagonalized; i.e. SOBI attempts to identify underlying sources that are as uncorrelated as possible through a given set of time lags. The resulting SOBI source components are then displayed on a grid and can easily be visualized and classified into source signals or biologic source contaminants. Respiration for instance in most subjects is a regular 10 to 20 cycles per minute signal that is distinct and has little spectral overlap with normal gastric slow wave signals with dominant frequency in the range of 2.5 to 4 cpm.
Researchers in Auckland University[9] recently quantified the effects of fat thickness and conductivity on resultant magnetic and electric fields using anatomically realistic, computer models. They showed that reduced fat conductivity did not change magnetic fields but significantly altered the patterns or waveforms and amplitudes of electric potentials. They further showed that 30 mm increase in thickness of the fat layer resulted in 10% decrease in amplitude of the magnetic fields, more than that of electric potentials, but magnetic field patterns were changed about four times less than electric potentials. Magnetic field patterns were preserved at 60% reduction in amplitude, and despite the 10% decrease in amplitude of the magnetic fields with a 30 mm increase in thickness of the fat layer, the ability to localize the underlying sources from the magnetic fields using surface current density measurements was only altered by less than 2 mm with their model. In other words, magnetic signals better preserve important slow wave characteristics with increased adiposity, geometry and reduced conductivity of interposing layers when compared to electrical signals. The Auckland University model clearly highlights some of the variations we would expect from MGG and EGG signal properties as a result of a change in geometry and conductivity of interposing tissue layers. A prior report on a large multicenter study involving 65 normal human subjects[30] demonstrated that subjects with a BMI>25 had a postprandial decrease in the percentage of gastric slow wave coupling compared to those with a BMI<25. Postprandial increases in EGG and MGG power spectral density have also been reported[8]. Our findings complement these reports though we did not observe changes in coupling during the postprandial state. In this study we compared the sensitivity of MGG and EGG detection of gastric slow waves and the corresponding dominant frequencies with simultaneously detected mucosal EMG among various BMI subpopulations of human subjects. Our results are consistent with prior reports and demonstrated that while subjects with BMI of ≥25 had significant reduction in post-prandial EGG sensitivity, a similar reduction in post-prandial MGG sensitivity was only observed in subjects with BMI of≥30. However, the application of the SOBI algorithm significantly improved both EGG and MGG sensitivity and neutralized these observed differences. Our group recently reported[31] a high cross-correlation between these signals and those simultaneously recorded by mucosal EMG with respect to their waveform and corresponding dominant frequency. The reported cross correlation also improved with application of the SOBI algorithm. This study, which had the largest BMI 51.3 and waist circumference of 53.5 inch was unable to demonstrate a threshold of anthropometric measurements that would impede SOBI identification of either MGG or EGG gastric slow waveforms or dominant frequencies.
We concluded that the magnetogastrogram is less affected than the electrogastrogram by changes in body mass index and waist circumference. The sensitivity of non-invasive gastric slow wave waveform and dominant frequency measurements is improved by use of SOBI signal analysis algorithm.
ACKNOWLEDGMENTS
The authors are grateful to the staff of General Clinical Research Center at Vanderbilt University Medical center for their assistance in this study. We are especially grateful to Linda Buck and Joan Kaiser for research nursing support throughout the duration of this study. This work was supported by a grant from the National Institutes of Health, USA (DK58697 to LAB). The authors in this paper:CO: performed the research, analyzed the data and wrote the paper; JE: performed the research and developed the script for signal analysis; SS: analyzed the data and designed some of the figures; TH: analyzed the data; EC: performed the research; WR: designed the experiment and edited the paper; LB: designed the experiment, performed the research and edited the paper.
Footnotes
Peer reviewer: Bronislaw L. Slomiany, Professor, Research Center, University of Medicine and Dentistry of New Jersey (UMDNJ)-NJ Dental School, 110 Bergen Street, Newark, NJ 07103-2400, the United States.
Contributor Information
Chibuike Obioha, Department of Surgery, Vanderbilt University, Nashville, TN, the United States.
Jon Erickson, Department of Physics & Astronomy, Vanderbilt University, Nashville, TN, the United States.
Somarajan Suseela, Department of Physics & Astronomy, Vanderbilt University, Nashville, TN, the United States.
Tahar Hajri, Department of Surgery, Vanderbilt University, Nashville, TN, the United States.
Eric Chung, Department of Physics & Astronomy, Vanderbilt University, Nashville, TN, the United States.
William Richards, Department of Surgery, University of South Alabama, Mobile, Alabama, the United States.
L. Alan Bradshaw, Department of Physics & Engineering, Lipscomb University, Nashville, TN, the United States; Department of Physics & Astronomy, Vanderbilt University, Nashville, TN, the United States.
REFERENCES
- 1.Alvarez WC, Mahoney LJ. Action Currents in Stomach and Intestine. Am J Physiol. 1922;58:476–493. [Google Scholar]
- 2.Bradshaw LA, Cheng LK, Richards WO, Pullan AJ. Surface Current Density Mapping for Identification of Gastric Slow Wave Propagation. Ieee T Bio-Med Eng. 2009;56:2131–2139. doi: 10.1109/TBME.2009.2021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du P, O'Grady G, Cheng LK, Pullan AJ. A Multiscale Model of the Electrophysiological Basis of the Human Electrogastrogram. Biophys J. 2010;99:2784–2792. doi: 10.1016/j.bpj.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhagen MAMT, Van Schelven LJ, Samsom M, Smout AJPM. Pitfalls in the analysis of electrogastrographic recordings. Gastroenterology. 1999;117:453–460. doi: 10.1053/gast.1999.0029900453. [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw LA, Ladipo JK, Staton DJ, Wikswo JP, Richards WO. The human vector magnetogastrogram and magnetoenterogram. Ieee T Bio-Med Eng. 1999;46:959–970. doi: 10.1109/10.775406. [DOI] [PubMed] [Google Scholar]
- 6.Bradshaw LA, Richards WO, Wikswo JP. Volume conductor effects on the spatial resolution of magnetic fields and electric potentials from gastrointestinal electrical activity. Med Biol Eng Comput. 2001;39:35–43. doi: 10.1007/BF02345264. [DOI] [PubMed] [Google Scholar]
- 7.Liang J, Chen JDZ. What can be measured from surface electrogastrography - Computer simulations. Digest Dis Sci. 1997;42:1331–1343. doi: 10.1023/a:1018869300296. [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw LA, Irimia A, Sims JA, Gallucci MR, Palmer RL, Richards WO. Biomagnetic characterization of spatiotemporal parameters of the gastric slow wave. Neurogastroent Motil. 2006;18:619–631. doi: 10.1111/j.1365-2982.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim JHK, Pullan AJ, Bradshaw LA, Cheng LK. Influence of body parameters on gastric bioelectric and biomagnetic fields in a realistic volume conductor. Physiol Meas. 2012;33:545–556. doi: 10.1088/0967-3334/33/4/545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monges H, Salducci J. A Method of Recording Gastric Electrical Activity in Man. Am J Dig Dis. 1970;15:271–276. doi: 10.1007/BF02233459. [DOI] [PubMed] [Google Scholar]
- 11.Erickson J, Obioha C, Goodale A, Bradshaw A, Richards W. Noninvasive detection of small bowel electrical activity from SQUID magnetometer measurements using SOBI. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1871–1474. doi: 10.1109/IEMBS.2008.4649550. [DOI] [PubMed] [Google Scholar]
- 12.Erickson JC, Obioha C, Goodale A, Bradshaw LA, Richards WO. Detection of Small Bowel Slow-Wave Frequencies From Noninvasive Biomagnetic Measurements. Ieee T Bio-Med Eng. 2009;56:2181–2189. doi: 10.1109/TBME.2009.2024087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belouchrani A, AbedMeraim K, Cardoso JF, Moulines E. A blind source separation technique using second-order statistics. Ieee T Signal Proces. 1997;45:434–444. [Google Scholar]
- 14.Brzana RJ, Koch KL, Bingaman S. Gastric myoelectrical activity in patients with gastric outlet obstruction and idiopathic gastroparesis. Am J Gastroenterol. 1998;93:1803–1809. doi: 10.1111/j.1572-0241.1998.00524.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith DS, Williams CS, Ferris CD. Diagnosis and treatment of chronic gastroparesis and chronic intestinal pseudo-obstruction. Gastroenterol Clin N. 2003;32:619-+. doi: 10.1016/s0889-8553(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 16.Qian LW, Pasricha PJ, Chen JDZ. Origins and patterns of spontaneous and drug-induced canine gastric myoelectrical dysrhythmia. Digest Dis Sci. 2003;48:508–515. doi: 10.1023/a:1022532515172. [DOI] [PubMed] [Google Scholar]
- 17.Bortoff A, Sillin LF. Changes in Intercellular Electrical Coupling of Smooth-Muscle Accompanying Atrophy and Hypertrophy. Am J Physiol. 1986;250:C292–C298. doi: 10.1152/ajpcell.1986.250.2.C292. [DOI] [PubMed] [Google Scholar]
- 18.Koch KL. Electrogastrography: physiological basis and clinical application in diabetic gastropathy. Diabetes Technol Ther. 2001;3:51–62. doi: 10.1089/152091501750220019. [DOI] [PubMed] [Google Scholar]
- 19.Madrid AM, Quera R, Defilippi C, et al. Gastrointestinal motility disturbances in Chagas disease. Rev Med Chile. 2004;132:939–946. doi: 10.4067/s0034-98872004000800005. [DOI] [PubMed] [Google Scholar]
- 20.Mintchev MP, Kingma YJ, Bowes KL. Accuracy of Cutaneous Recordings of Gastric Electrical-Activity. Gastroenterology. 1993;104:1273–1280. doi: 10.1016/0016-5085(93)90334-9. [DOI] [PubMed] [Google Scholar]
- 21.Mintchev MP, Bowes KL. Impact of external factors on the stability of human electrogastrograms. Med Biol Eng Comput. 1996;34:270–272. doi: 10.1007/BF02520087. [DOI] [PubMed] [Google Scholar]
- 22.Allescher HD, Abraham-Fuchs K, Dunkel RE, Classen M. Biomagnetic 3-dimensional spatial and temporal characterization of electrical activity of human stomach. Digest Dis Sci. 1998;43:683–693. doi: 10.1023/a:1018852208687. [DOI] [PubMed] [Google Scholar]
- 23.Bradshaw LA, Myers A, Wikswo JP, Richards WO. A spatio-temporal dipole simulation of gastrointestinal magnetic fields. Ieee T Bio-Med Eng. 2003;50:836–847. doi: 10.1109/TBME.2003.813549. [DOI] [PubMed] [Google Scholar]
- 24.Turnbull GK, Ritcey SP, Stroink G, Brandts B, van Leeuwen P. Spatial and temporal variations in the magnetic fields produced by human gastrointestinal activity. Med Biol Eng Comput. 1999;37:549–554. doi: 10.1007/BF02513347. [DOI] [PubMed] [Google Scholar]
- 25.Richards WO, Bradshaw LA, Staton DJ, et al. Magnetoenterography (MENG) - Noninvasive measurement of bioelectric activity in human small intestine. Digest Dis Sci. 1996;41:2293–2301. doi: 10.1007/BF02100117. [DOI] [PubMed] [Google Scholar]
- 26.Bradshaw LA, Irimia A, Sims JA, Richards WO. Biomagnetic signatures of uncoupled gastric musculature. Neurogastroent Motil. 2009;21:778–E50. doi: 10.1111/j.1365-2982.2009.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obesity. 2004;28:1018–1025. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 28.Asayama K, Dobashi K, Hayashibe H, et al. Threshold values of visceral fat measures and their anthropometric alternatives for metabolic derangement in Japanese obese boys. Int J Obesity. 2002;26:208–213. doi: 10.1038/sj.ijo.0801865. [DOI] [PubMed] [Google Scholar]
- 29.Kim JHK, Bradshaw LA, Pullan AJ, Cheng LK. Characterization of Gastric Electrical Activity Using Magnetic Field Measurements: A Simulation Study. Ann Biomed Eng. 2010;38:177–186. doi: 10.1007/s10439-009-9804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonian HP, Panganamamula K, Parkman HP, et al. Multichannel electrogastrography (EGG) in normal subjects: A multicenter study. Digest Dis Sci. 2004;49:594–601. doi: 10.1023/b:ddas.0000026304.83214.50. [DOI] [PubMed] [Google Scholar]
- 31.Somarajan S, Muszynski ND, Obioha C, Richards WO, Bradshaw LA. Biomagnetic and bioelectric detection of gastric slow wave activity in normal human subjects-a correlation study. Physiol Meas. 2012;33:1171–1179. doi: 10.1088/0967-3334/33/7/1171. [DOI] [PMC free article] [PubMed] [Google Scholar]