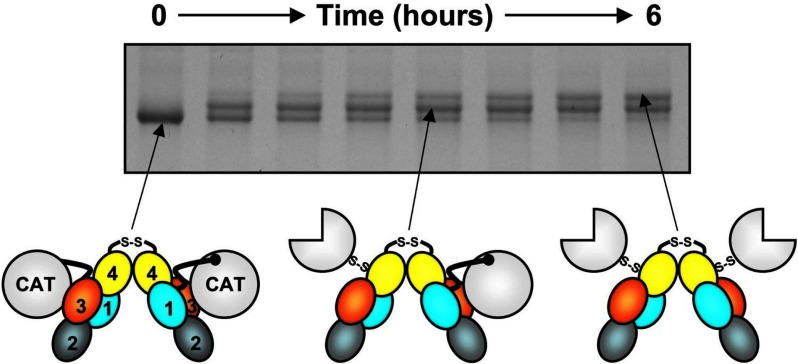
Figure 5.
FXI activation. Each FXI subunit is activated by cleavage between Arg369 and Ile370. FXI (0-hour time point) migrates slightly faster than FXIa (top band, 6 hours) on nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Activation of FXI (schematic at left) by α-thrombin (shown) or FXIIa proceeds through an intermediate with 1 cleaved subunit (center), designated 1/2-FXIa. Subsequent conversion of 1/2-FXIa to fully activated FXIa (right) appears to be a slower process. In the diagrams, the A1, A2, A3, and A4 domains are shown in light blue, cyan, orange, and yellow, respectively, and the catalytic domains (CAT) are in white. Circles represent unactivated catalytic domains; three-fourths circles, activated catalytic domains. In these diagrams, the catalytic domain moves relative to the apple domain disk after cleavage of Arg369-Ile370, exposing a surface on A3 thought to contain a FIX-binding site.