Abstract
GNAL was identified as a cause of dystonia in patients from North America, Europe and Asia. In this study, we aimed to investigate the prevalence of GNAL variants in Brazilian patients with dystonia. Ninety-one patients with isolated idiopathic dystonia, negative for THAP1 and TOR1A mutations, were screened for GNAL variants by Sanger sequencing. Functional characterization of the Gαolf protein variant was performed using the bioluminescence resonance energy transfer assay. A novel heterozygous nonsynonymous variant (p.F133L) was identified in a patient with cervical and laryngeal dystonia since the third decade of life, with no family history. This variant was not identified in healthy Brazilian controls and was not described in 63000 exomas of the ExAC database. The F133L mutant exhibited significantly elevated levels of basal BRET and severely diminished amplitude of response elicited by dopamine, that both indicate su bstantial functional impairment of Gαolf in transducing receptor signals, which could be involved in dystonia pathophysiology. GNAL mutations are not a common cause of dystonia in the Brazilian population and have a lower prevalence than THAP1 and TOR1A mutations. We present a novel variant that results in partial Gαolf loss of function.
Keywords: dystonia, genetics, GNAL, DYT25, Gαolf
INTRODUCTION
Dystonia is a hyperkinetic movement disorder characterized by sustained or intermittent muscle contractions leading to abnormal movements and/or postures [1]. According to the latest consensus, the term “isolated dystonia” refers to a phenomenological characteristic where dystonia is the only motor feature, with the exception of tremor, and has no implications for the etiology [1]. In the etiology axis, the consensus defined three categories: inherited (dystonia forms of proven genetic origin), acquired (dystonia due to a known specific cause, except for genetic causes) and idiopathic (dystonia of unknown cause, including sporadic and familial cases). Dystonias initially classified as idiopathic can be reclassified as inherited, once causal genes are discovered. To date, several genes have been associated with isolated dystonia [2]. Mutations in THAP1 and TOR1A are well-known causes of this disorder and have been characterized in Brazilian patients [3]. The progress of next generation sequencing (NGS) technologies has led to the identification of new genes associated with this disorder. GNAL was recently identified as a causative gene for dystonia [4].
GNAL encodes α subunit of the stimulatory G protein (Gαolf) with prominent expression in the striatum where it forms heterotrimeric complex with G protein βγ subunits [5]. This complex couples dopamine D1 receptors in the region to stimulation of adenylyl cyclase type 5 that produces second messenger cAMP. Disruption of Gαolf can lead to impaired dopaminergic signal transduction in the striatum [5–7].
The prevalence of GNAL mutations in dystonia was initially reported as 15% in a study that analyzed specific families [4]. However, studies conducted in European and Asian populations reported frequencies below 2.6% [8–10]. Therefore, it is necessary to collect more data in order to determine the prevalence of GNAL variants, as well as to report novel variants with pathogenic potential in different populations.
In this study, we aimed to investigate the prevalence of GNAL variants as the cause of dystonia in a group of Brazilian patients. We identified a novel variant of the gene in a patient with dystonia and showed that it alters Gαolf function.
METHODS
Patients
Ninety-one patients with idiopathic isolated dystonia were recruited by movement disorders specialists participating in the project “Brazilian Network for the study of dystonia” approved by the institutional review board. Informed written consent was obtained from all participants. They have been screened in a previous study and were negative for mutations in TOR1A and THAP1 [3]. Table 1 lists the patients’ clinical characteristics.
Table 1.
Clinical characteristics of the probands with dystonia.
| Sex | Male: 30 (33%) |
| Female: 61 (67%) | |
| Age at exam | Mean: 48.7 years (SD± 17.5years) |
| Range: 2 to 84 years | |
| Dystonia age of onset | Mean: 32.5 years (SD± 18.1 years) |
| Range: 3 months to 68 years | |
| Median: 29 years | |
| Age of onset Distribution | Infancy (0 – 2 years):2 (2.2%) |
| Childhood (3 – 12 years): 15 (16.5%) | |
| Adolescence (13 – 20 years): 11 (12.1%) | |
| Early adulthood (21 – 40 years): 31(34%) | |
| Late adulthood (> 40 years): 32 (35.2%) | |
| Family history | Negative: 67 (73.6%) |
| Positive: 11 (12.1%) | |
| Possible*: 10 (11%) | |
| Not informed: 3 (3.3%) | |
| Dystonia Distribution | Focal: 39 (42.8%) |
| Segmental: 34 (37.4%) | |
| Generalized: 13 (14.3%) | |
| Multifocal: 5 (5.5%) |
Subjects reported family history of dystonia but family members were not examined for confirmation.
Molecular Analysis
DNA was extracted from peripheral blood using the Gentra Puregene® Blood DNA Kit (Qiagen) according to the manufacturer’s instructions. GNAL variants were screened in the 12 exons and splice sites of the isoforms NM_182978 and NM_001142339 by Sanger sequencing on an ABI 3500 XL Genetic Analyzer (Applied Biosystems) following standard protocols. Primer sequences and PCR conditions were similar to those described by Fuchs et al. [4], except for exon 1 of NM_001142339 and for exon 2 (1F-gaaacaattctcgtgtaaaaag/1R-tagccgggtctgagcaatc and 2F-attgctcagacccggctag/2R-gaatgtctactccattatc).
Whenever nonsynonymous variants were identified in the patients, allelic frequencies were investigated in 234 chromosomes of healthy Brazilian controls and in a population database of 63000 exomas (ExAC – Exome Aggregation Consortium). In silico analysis was performed using PolyPhen-2, SIFT, Mutation Taster, PROVEAN and CADD scores. The degree of amino acid conservation among species was accessed through the ClustalW2 program (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Functional characterization was performed using the improved bioluminescence resonance energy transfer (BRET) assay [11] similar in design and concept to the assay previously used to evaluate the effect of mutations in Gαolf [4]. Briefly, constructs encoding dopamine D1 receptor, Gαolf, Venus155–239–Gβ1, Venus1–155–Gγ2, masGRK3ct-Nluc, PTX-S1 and Flag-tagged Ric-8B were transfected into HEK293/17 cells at a 1:6:1:1:1:1:1 ratio, with 7.5 μg of total DNA delivered per 3.5 × 106 cells. After 16 h, cells were stimulated with 100 μM dopamine followed by treatment with 100 μM of D1 receptor antagonist SCH39166. One-way ANOVA followed by Tukey’s post-hoc test was performed to determine statistically significant differences relative to wild-type control.
RESULTS
We identified four synonymous variants (c.66C>T, c.132C>T, c.1014C>T and c.1056C>T) in six probands and a missense variant c.399C>A (p.F133L) in a single proband (numbering is based on NM_001142339 and NP_001135811).
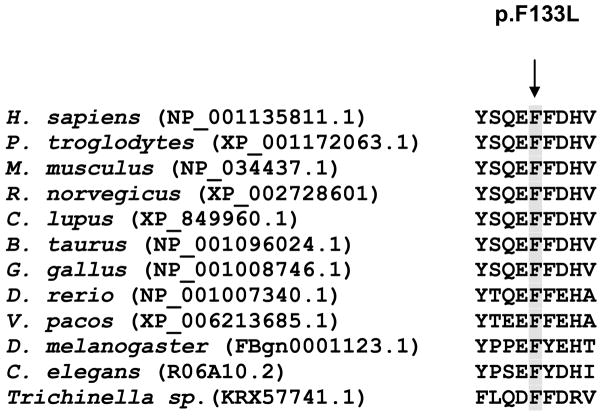
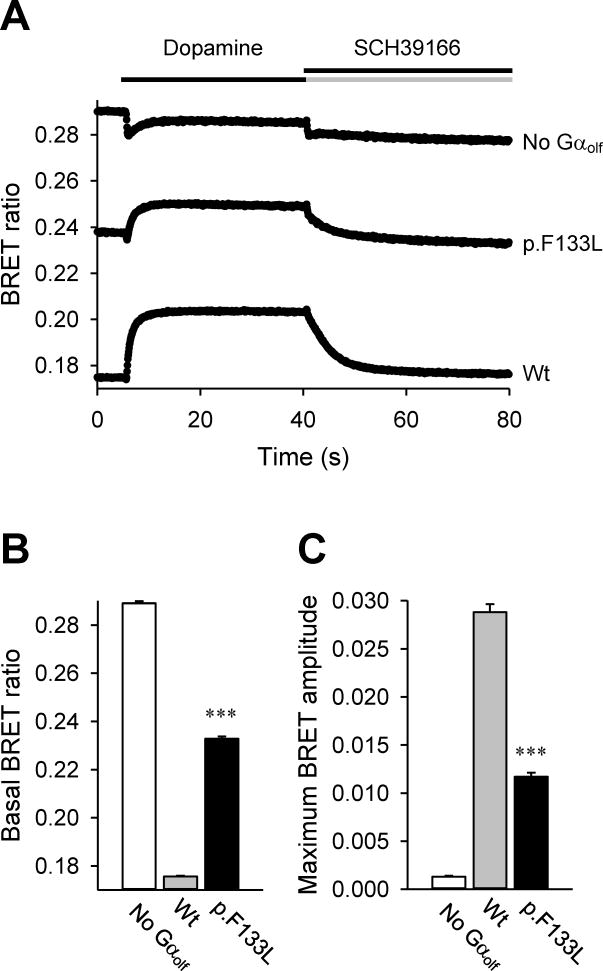
The variant p.F133L was identified in a 59 year-old female patient with cervical and laryngeal dystonia since the age of 23, with no family history. She was responsive to botulinum toxin. This variant was neither found in the 234 chromosomes of the normal controls nor among the 63000 exomas of the ExAC database (November, 2015). Protein alignment in ClustalW showed that phenylalanine at position 133 is well conserved throughout the species (Figure 1). The SIFT algorithm predicted that this variant is tolerated but in silico analyses using the Mutation Taster, PolyPhen-2 and PROVEAN algorithms predicted that it is detrimental to protein function. The scaled CADD score for this variant is 26.6, suggesting it is deleterious. To investigate the functional consequences we examined the impact of F133L mutation on the activation of Gαolf by D1R receptors using the BRET assays (Figure 2). In this system, application of dopamine triggers Gαolf dissociation from Gβγ subunits producing BRET signal which is quenched by subsequent antagonist application. The F133L mutant exhibited significantly elevated levels of basal BRET and severely diminished amplitude of response elicited by dopamine, that both indicate substantial functional impairment of Gαolf in transducing receptor signals. Despite significant reduction in functional activity, mutant Gαolf maintained considerable functional activity leading us to conclude that the mutation causes a partial loss of function.
Figure 1.
Cross-species protein sequence alignment of Gαolf using ClustalW2.
Phenylalanine at position 133 is highly conserved.
Figure 2. Functional effects of F133L substitution in Gαolf assessed by the Bioluminescence resonance energy transfer (BRET) assay.
A) Time course of Golf activation in D1R-transfected cells. Responses to dopamine and SCH39166 (D1R selective antagonist) were monitored. Variations of p.F133L from wild-type Gαolf (wt) in the BRET signal indicate impaired function of the mutant. Representative traces are shown (n = 6). B) High basal BRET signal from p.F133L mutant before dopamine application suggests impairment of Gαolf/Gβγ trimer formation. C) Unlike the wild-type protein (wt), the altered form (p. F133L) showed small response to dopamine application, which is consistent with impaired association with the Gβγ dimer. Values shown in B and C represent mean ± SEM from three independent experiments. One-way ANOVA followed by Tukey’s post-hoc test was performed to determine statistically significant differences relative to wild-type control. Triple asterisk (***) indicates P < 0.001, t-test.
DISCUSSION
In this study, we investigated GNAL variants in a group of Brazilian patients with idiopathic dystonia who were negative for THAP1 and TOR1A mutations and identified a novel putative pathogenic variant (p.F133L). The prevalence of disruptive GNAL mutations in this cohort of dystonia patients was 1.09%, which is consistent with those recently reported between 0.5% and 2.6% among individuals of European and Asian ancestry [8–11]. In Brazilian patients, idiopathic dystonia associated to GNAL mutations is less frequent than dystonia associated to THAP1 (8.8%) or TOR1A (2.6%) mutations [3].
Dystonia caused by mutations in THAP1, TOR1A and GNAL differ in terms of clinical characteristics, such as age of onset, site of onset and progression. Several studies indicate that phenotype is a major predictor for genotype, however, there is some degree of heterogeneity, and less typical cases affected by mutations in these genes may have overlapping characteristics [3,4,12]. The typical TOR1A phenotype is characterized by early-onset (mean 13 years) limb dystonia (arms and legs equally affected) which frequently generalizes. Spread to cranial muscles is less common [3]. In THAP1 mutations symptomatic carriers, the most frequent phenotype is early-onset dystonia (mean 16 years) initially in the arm or craniocervical region, legs are rarely the site of onset. Approximately half of the cases progress to multifocal/generalized forms. Dysphonia is also an important feature in more than two thirds of the cases [3]. The typical GNAL patient presents with adult-onset (mean 32 years) segmental dystonia (craniocervical), the neck is the most common site of onset, arm onset is rare, dystonia remains focal/segmental in most of the cases, speech is affected in approximately 44%, leg involvement is not common [4, 10, 12]. The patient carrying the p.F133L variant was of Italian and Portuguese ethnicity and a sporadic case with a typical phenotype of cervical dystonia developing the disease in the third decade of life. As we did not have access to the parents’ DNA, we could not confirm whether the absence of a family history was due to a de novo mutation or incomplete gene penetrance. Other unaffected family members were not available to perform a segregation analysis, this information would have conclusively supported the pathogenic role of this variant. The functional assay revealed deficits in Gαolf coupling to D1R receptor. It has been demonstrated that the levels of Gαolf directly influence both basal and D1R-activated cAMP production in the striatum [5]. Thus, reduction in Gαolf function is expected to result in the reduction of adenylyl cyclase activation. Although we did not perform further functional studies, we can speculate that Gαolf variant F133L ultimately contributes to the disease phenotype by impairing coupling of neurotransmitter receptor signaling to cAMP production in the striatal neurons.
This is the first study to investigate GNAL variants in patients from Brazil. Our data confirm GNAL mutations as a cause of dystonia in this sample of patients with a lower prevalence than THAP1 and TOR1A mutations. The newly identified GNAL variant contributes to our understanding of this debilitating disorder. Future studies should be conducted to elucidate the mechanisms by which mutations in this gene cause dystonia in order to identify ideal therapeutic targets.
Acknowledgments
Study funding: Sao Paulo Research Foundation (FAPESP) grants # 2010/19206-0 (PCA); 2011/18202-3 (COS); 2013/09867-7(COS), 2014/17128-2(PCA) NIH grant NS081282 (KAM). The authors thank all patients and their families, the Exome Aggregation Consortium and the groups that provided exome variant data for comparison. A full list of contributing groups can be found at http://exac.broadinstitute.org/about.
Footnotes
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. MovDisord. 2013;28(7):863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozelius LJ, Lubarr N, Bressman SB. Milestones in dystonia. Mov Disord. 2011;26(6):1106–26. doi: 10.1002/mds.23775. [DOI] [PubMed] [Google Scholar]
- 3.da Silva FP, Junior, dos Santos CO, Silva SM, et al. Novel THAP1 variants in Brazilian patients with idiopathic isolated dystonia. J Neurol Sci. 2014;344(1–2):190–192. doi: 10.1016/j.jns.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs T, Saunders-Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat genet. 2013;45(1):88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hervé D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat. 2011;5:48. doi: 10.3389/fnana.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corvol JC, Studler JM, Schonn JS, Girault JA, Hervé D. Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76(5):1585–1588. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 7.Vemula SR, Puschmann A, Xiao J, et al. Role of Gα(olf) in familial and sporadic adult-onset primary dystonia. Hum Mol Genet. 2013;22(12):2510–2519. doi: 10.1093/hmg/ddt102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erro R, Bhatia KP, Hardy J. GNAL mutations and dystonia. JAMA Neurol. 2014;71(8):1052–1053. doi: 10.1001/jamaneurol.2014.1506. [DOI] [PubMed] [Google Scholar]
- 9.Charlesworth G, Bhatia KP, Wood NW. No pathogenic GNAL mutations in 192 sporadic and familial cases of cervical dystonia. MovDisord. 2014;29(1):154–155. doi: 10.1002/mds.25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar KR, Martemyanov KA, Lohmann K. GNAL mutations and dystonia--reply. JAMA Neurol. 2014;71(8):1053–1054. doi: 10.1001/jamaneurol.2014.1509. [DOI] [PubMed] [Google Scholar]
- 11.Masuho I, Martemyanov KA, Lambert NA. Monitoring G Protein Activation in Cells with BRET. Methods Mol Biol. 2015;1335:107–113. doi: 10.1007/978-1-4939-2914-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders-Pullman R, Fuchs T, San Luciano M, et al. Heterogeneity in primary dystonia: lessons from THAP1, GNAL, and TOR1A in Amish-Mennonites. MovDisord. 2014;29(6):812–818. doi: 10.1002/mds.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]