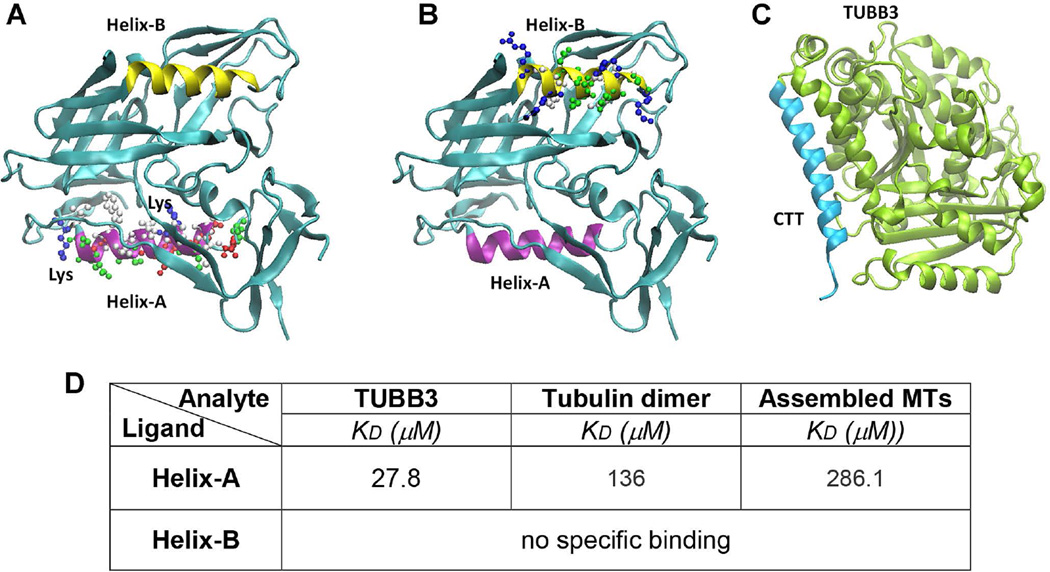
Figure 2. 3D structure of gp120 and TUBB3.
The tridimensional structures of gp120 (A and B) show that two helices in gp120 (Helix-A in purple and Helix-B in yellow) occupied two opposite sides and both could be contributing to the effective binding to the CTT (C, light blue) of TUBB3. Furthermore, we indicated the lysine residues present in Helix-A that could be responsible in the stabilization of helix-helix interaction between Helix-A and CTT. D. BiacoreT200 was used to determine the kinetic parameters for the binding of recombinant TUBB3, tubulin dimer, and assembled MTs Helix-A and HeliX-B peptides. Peptides were injected at different concentrations (10 nM – 500 µM). Data are from three independent experiments.