Abstract
Candida albicans (CA) is a kind of fungi that can cause high morbidity and mortality in immunocompromised patients. However, preventing CA infection in these patients is still a daunting challenge. Herein, inspired from the fact that immunization with Secreted aspartyl proteinases 2 (Sap2) could prevent the infection, we propose to use filamentous phage, a human-safe virus nanofiber specifically infecting bacteria (~900 nm long and 7 nm wide), to display an epitope peptide of Sap2 (EPS, with a sequence of Val-Lys-Tyr-Thr-Ser) on its side wall and thus serve as a vaccine for preventing CA infection. The engineered virus nanofibers and recombinant Sap2 (rSap2) are then separately used to immunize mice. The humoral and cellular immune responses in the immunized mice are evaluated. Surprisingly, the virus nanofibers significantly induce mice to produce strong immune response as rSap2 and generate antibodies that can bind Sap2 and CA to inhibit the CA infection. Consequently, immunization with the virus nanofibers in mice dramatically increase the survival rate of CA-infected mice. All these results, along with the fact that the virus nanofibers can be mass-produced by infecting bacteria cost-effectively, suggest that virus nanofibers displaying EPS can be a vaccine candidate against fungal infection.
Keywords: fungal infection, viruses, nanofibers, vaccine
1. Introduction
C. albicans (CA) is well known as an opportunistic fungus existing in normal organisms, but could cause superficial and systemic infections in immunocompromised or debilitated hosts such as patients with cancer and AIDS. Though superficial CA infections are non-lethal, systemic candidiasis infections result in high modality and mortality in mildly immunocompromised individuals even with antifungal therapy.[1, 2] [3] During the past decades, therapeutic antifungals have been widely used against candidiasis, dramatically increasing the drug tolerance and resistance.[4] Hence, there is a pressing need in the development of new vaccines against candidiasis at the infectious stage.
Subunit vaccines, which consist of one or more proteins conjugated with a protein carrier to acquire sufficient immunogenicity, are the most studied types of fungal vaccines and most likely to result in an approvable vaccine.[5] There are several virulence factors available and helpful for CA infection.[6, 7] Among them, the secretory aspartyl proteinases (Saps) family (Sap1–10) was considered as the major determinants and related to several putative virulence attributes such as hyphal formation, adhesion, phenotypic switching, dimorphism, and the secretion of hydrolytic enzymes in systemic infections.[8–11] Sap2 is the most abundant form of Saps that cause the damage and virulence due to the infection.[8, 12, 13] Furthermore, it was also found that antibodies against Sap2, which were induced by immunization with Sap2 or reconstructive Sap2, had a protective role against CA infection in rats or mice.[14–17] These results suggested that the Sap2 based subunit vaccine might be a kind of valuable vaccines against candidiasis. A very short epitope peptide of Sap2 (EPS, with a sequence of Val-Lys-Tyr-Thr-Ser) was demonstrated to have the ability to respond to IgG from candidiasis infected patients.[18–20] This discovery indicated that the EPS might be the immunodominant epitope of Sap2 for developing potential vaccines against CA infection. Hence, we propose to use protein-based phage nanofibers to display and thus carry the EPS to replace the Sap2 in immunotherapy of the fungal infection (Scheme 1).
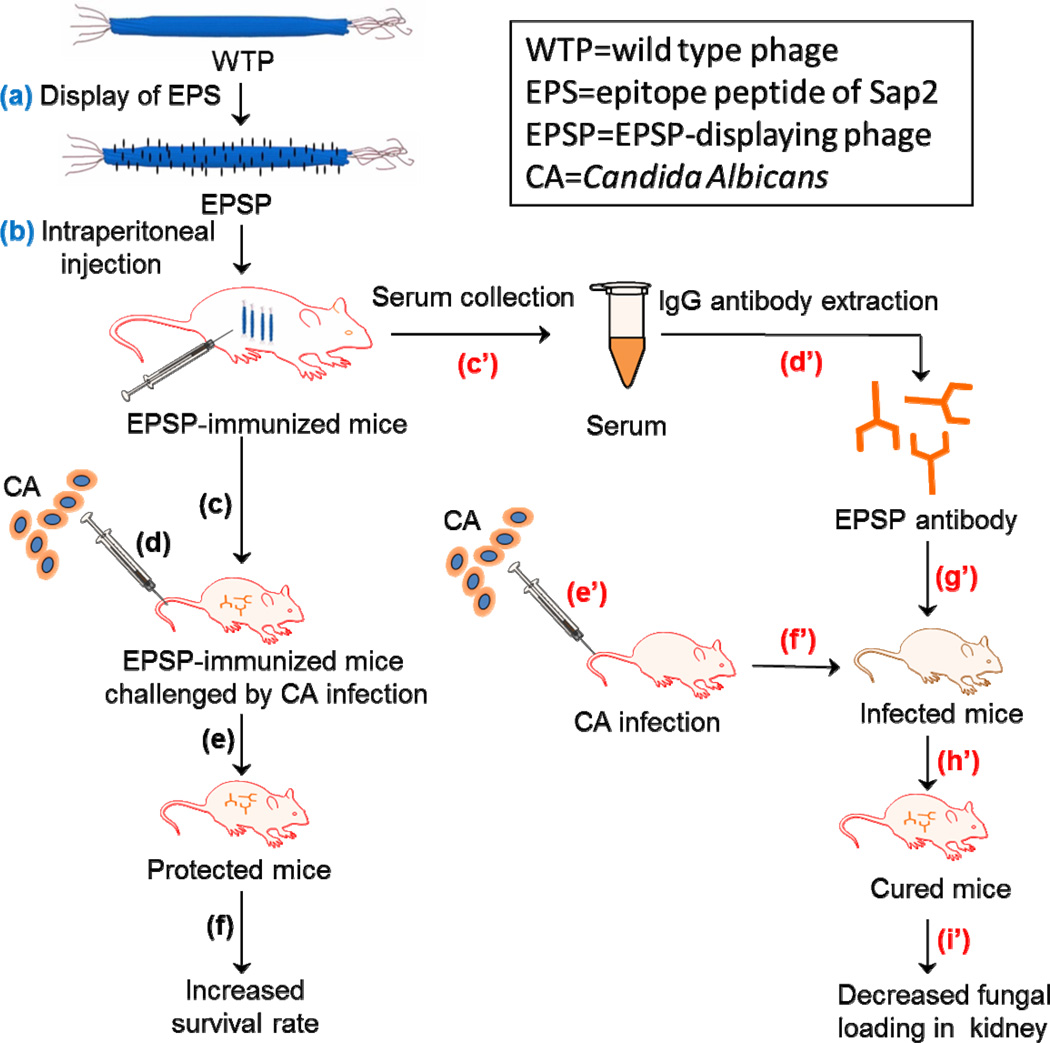
Scheme 1.
Schematic illustration of the general idea using EPSP nanofibers (~900 nm long and ~7 nm wide) as a vaccine for preventing CA infection. Firstly, EPS was displayed on the WTP to form EPSP nanofibers (a), which were intraperitoneally injected into the mice for three times (25 µg/mice each time) to obtain EPSP-immunized mice (b). Then, two approaches were adopted to prove the use of EPSP as a vaccine for preventing CA infection. The first approach is to test if CA cells can infect the EPSP-immunized mice. Briefly, one week after the last mmunization, mice were challenged (c) by injecting 106 CA cells through tail vein (d). It was found that the EPSP-immunized mice were protected from CA infection (e), as evidenced by the decreased fungal loading in kidney, less visceral lession and increased survival rate (f). The second approach is to test if EPSP antibodies collected from the EPSP-immunized mice can be used to cure the CA-infected mice. Briefly, serum was collected from the blood of EPSP-immunized mice (c’), followed by the extraction of IgG from the serum to obtain EPSP antibodies (d’). After the mice were infected by CA cells through tail vein injection (e’ and f’), the EPSP antibodies were injected intravenously into the infected mice (g’), which led to the curing of CA infection (h’) as evidenced by the significantly reduced fungal loading in kidneys (i).
Filamentous phage is a nanofiber-like virus (~900 nm long and 7 nm wide) that specifically infects bacteria.[21–23] It is made of DNA and proteins.[24, 25] The DNA is encapsulated by a coat made of five structural proteins, including one major coat protein (pⅧ) constituting the side wall of the nanofibers and four minor coat proteins with two of them each constituting one distal tip of the nanofibers.[26–28] Filamentous phage increasingly attracts scientists’ attention in recent years because of its wide usage in many fields. For example, it can act as a template for nanomaterials formation[29–34], as a probe for sensing and imaging[35, 36], as a vector for targeted drug and gene delivery[27, 28, 37], as a platform for screening peptides or antibodies[25] and as a scaffold for inducing stem cell differentiation and bone formation[38–40].
A foreign peptide can be fused to the N-terminal end of pⅧ by genetic means without interfering with the packaging of coat protein and DNA into mature phage nanofibers.[41] The peptide displayed on the phage, if it was an epitope derived from a native functional protein, was found to adopt a conformation similar to that found in the native protein.[42] Hence, in this study we displayed EPS on the side wall (termed major coat) of virus nanofibers by fusion of EPS to the solvent-exposed N-terminal of the major coat protein (pⅧ, ~3000 copies) constituting the side wall of phage (Scheme 1). We then proceeded to evaluate the protective effect of EPS-displaying phage (EPSP) nanofibers as a subunit vaccine against candidiasis (Scheme 1). The original protein, a recombinant Sap2 (rSap2) bearing the EPS was used as a control. Both the EPSP and rSap2 were used to immunize the mice. Then the humoral and cellular immune responses to the EPSP nanofibers in the immunized mice were investigated along with the responses to the rSap2. A series of assays, including the survival rate, fungal loading in kidneys, and pathological change in kidneys and livers against CA infection in BALB/c mice, were used to assess the protective role of the EPSP nanofibers (Scheme 1). In the end, the antibody generated in response to the EPSP nanofibers was passively inoculated to CA-infected mice to further prove the protective role of the EPSP nanofibers as a subunit vaccine (Scheme 1).
2 Results
2.1. Characterization of purified rSap2 protein and EPSP nanofibers
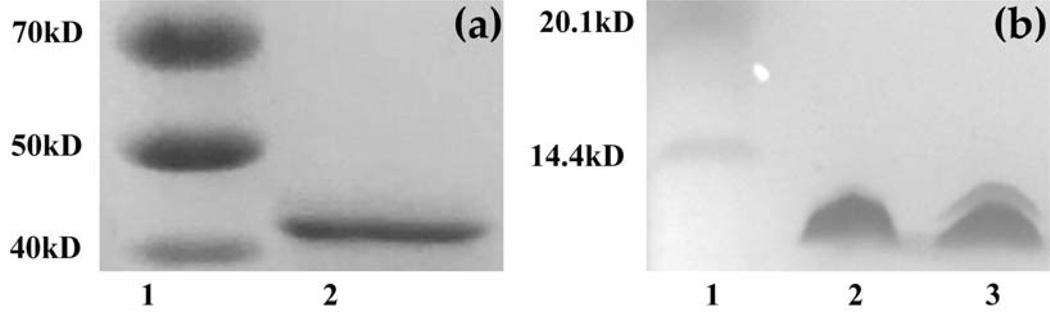
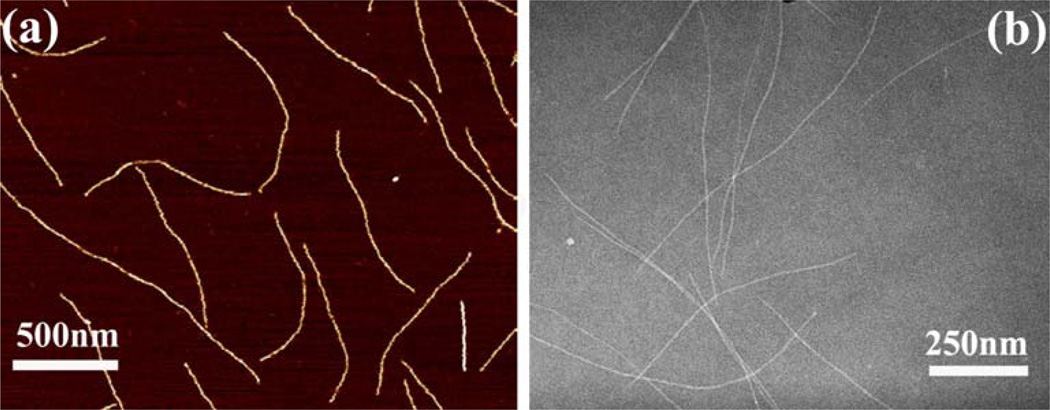
rSap2 was purified with Ni affinity purification procedure. Sap2 is a protein with a molecular weight of about 43 kDa (Figure 1a). SDS-PAGE confirms the purification of the rSap2 (Figure 1a). pⅧ protein constituting the side wall of engineered phage (i.e., with EPS fused to the solvent-exposed terminal of the pⅧ) or wild type phage (WTP) nanofibers was separated by SDS-PAGE (Figure 1b). SDS-PAGE (Figure 1b) demonstrated that the EPS was successfully fused to the pⅧ of WTP to achieve peptide display on the side wall of the WTP, forming EPSP nanofibers. AFM and TEM images collectively confirm that the EPSP is indeed a nanofiber (~900 nm long and 7 nm wide) (Figure 2).
Figure 1.
SDS-PAGE to analyze rSap2 and EPSP. (a) Purified rSap2 protein. Sap2 was identified with a molecular weight of about 43 kDa. Lane1, Marker; Lane 2, rSap2 protein, (b) Purified EPSP. It showed that EPS was successfully fused to the wild type pⅧ of phage. Lane1, Maker, Lane 2, WTP; Lane3, EPSP.
Figure 2.
AFM (a) and TEM (b) image of EPSP nanofibers. These images show that the EPSP nanofibers were about ~900 nm long. Some shorter nanofibers, which are the broken peieces by AFM tips, are seen in AFM.
2.2. Antibody response against rSap2 and CA
2.2.1. Western blotting
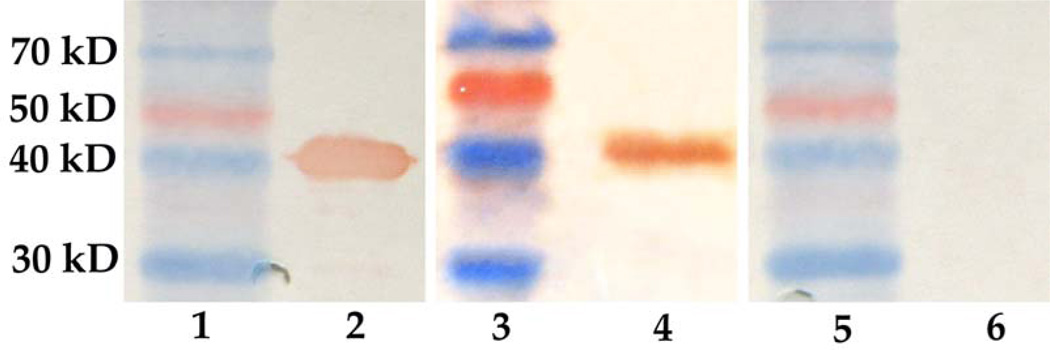
The antibody response against rSap2 was assessed by Western blotting. rSap2 could bind to the antibodies in the sera collected from the mice immunized with the rSap2 (Figure 3, lane 2) and EPSP nanofibers (Figure 3, lane 4), but not with the sera from the mice immunized with the WTP nanofibers (Figure 3, lane 6). These results indicate that the antibodies generated due to the immunization with EPSP nanofibers could bind to Sap2.
Figure 3.
One week after the third time immunization, sera from each group of immunized mice were collected and used for western blotting assay. Lane 1, 3 and 5, marker; lane 2, rSap2 + sera from mice immunized with rSap2; lane 4, rSap2 + sera from mice immunized with EPSP nanofibers; lane 6, rSap2 + sera from mice immunized with WTP nanofibers. These results indicate that rSap2 can only react with the sera from mice immunized with rSap2 or EPSP nanofibers.
2.2.2. Immunofluorescence
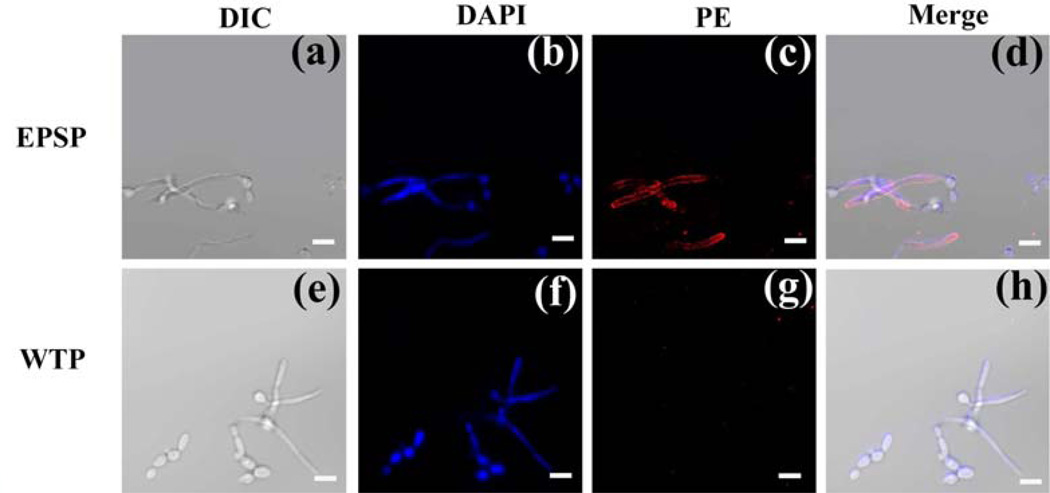
To make sure that the reactivity of anti-EPSP antibody with Sap2 was not attributable to the contamination produced during prokaryotic expression, immunofluorescence assay was done to estimate the antibody response to CA. The ability of anti-EPSP antibody in the sera to bind the Sap2-producing CA could be seen visually from the image (Figure 4c), while the control group of anti-WTP antibody in the sera did not bind CA (Figure 4g). These data confirm that the EPSP nanofibers acted as an antigen and stimulated mice to produce antibodies that can bind Sap2-producing CA. The binding of CA might prevent the adhesion and migration of CA, which will relieve the CA infection.[17]
Figure 4.
Immunofluorescence to assess the binding affinity of antibody-containing sera with CA. (a) Morphology of CA under differential interference contrast (DIC) microscope; (b) CA stained with DAPI (excitation: 358nm, emission: 461nm), (c) CA incubated with sera containing anti-EPSP and stained by goat anti-rabbit IgG conjugated with PE(excitation: 495nm, emission: 519nm), (d) Merge of a, b and c, (e) Morphology of CA under DIC microscope, (f) CA stained with DAPI(excitation: 358nm, emission: 461nm), (g) CA incubated with sera containing anti-WTP and stained by goat anti-rabbit IgG conjugated with PE(excitation: 488nm, emission: 575nm), (h) Merge of e, f and g. The fluorescence images illustrated that the antibodies in sera collected from mice immunized with EPSP could bind to CA. Scale bars: 5µm
2.3. Cellular immunologic response
2.3.1. Cytokines production induced by EPSP nanofibers
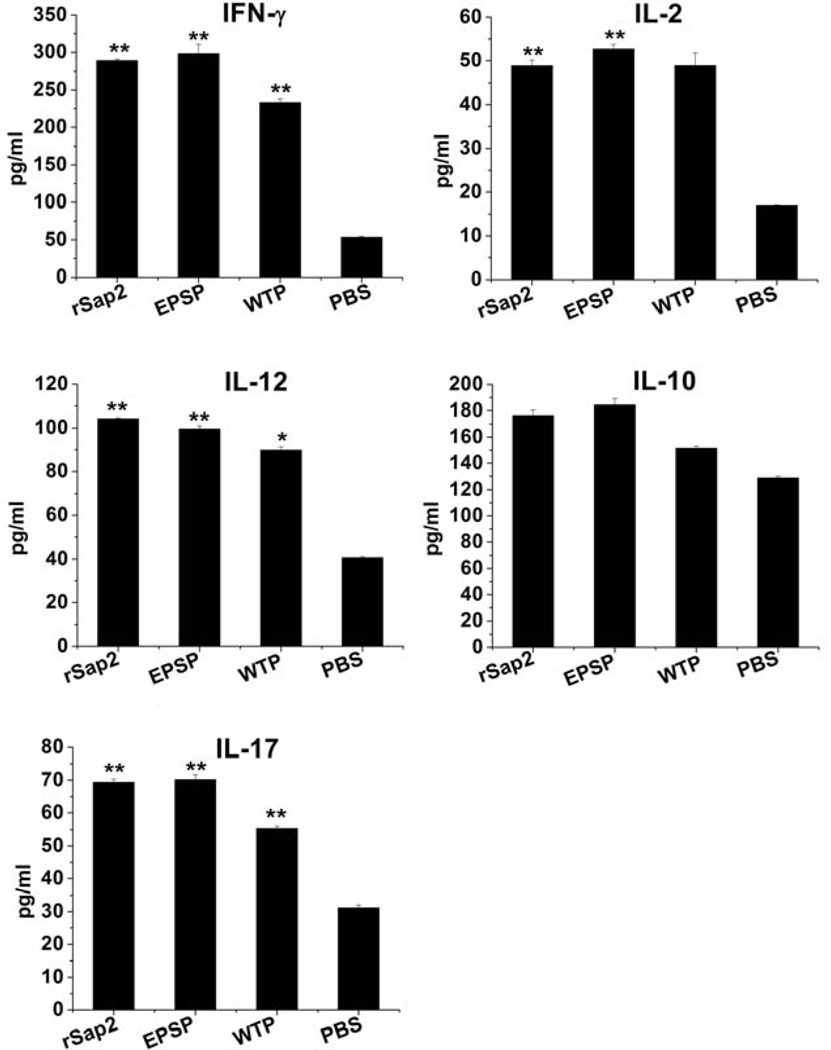
To assess the cellular response, the cytokines levels, such as the production of IFN-γ, IL-2, IL- 10, IL-12 and IL-17 were evaluated in the immunized mice. Our results showed that the amount of all the cytokines except IL-10 in the mice immunized with the EPSP and WTP nanofibers as well as rSap2 was significantly higher than that in the mice injected with phosphate-buffered saline (PBS) (p < 0.05). These data suggest that both viral nanofibers and rSap2 could induce Th1 and TH17 type cytokines gene expression (Figure 5).
Figure 5.
Cytokines expression in the supernatants of splenocytes culture. One week after the last immunization, spleens of immunized mice were removed, ground and seeded in 96 wells plates, followed by stimulating with rSap2, EPSP, WTP or PBS. Then the production of cytokines was measured. Group 1, rSap2 immunized mice; group 2, EPSP immunized mice; group 3, WTP immunized mice; group; 4, PBS injected mice. These data suggest that both phage nanofibers and rSap2 could induce Th1 (IFN-γ, IL-2, IL-12)and TH17(IL-17) type cytokines gene expression, but not TH2(IL-10)(p>0.05). All data was compared to PBS group, * Significant (p<0.05), ** Very significant (p<0.01).
2.3.2. Delayed-type hypersensitivity (DTH) reaction
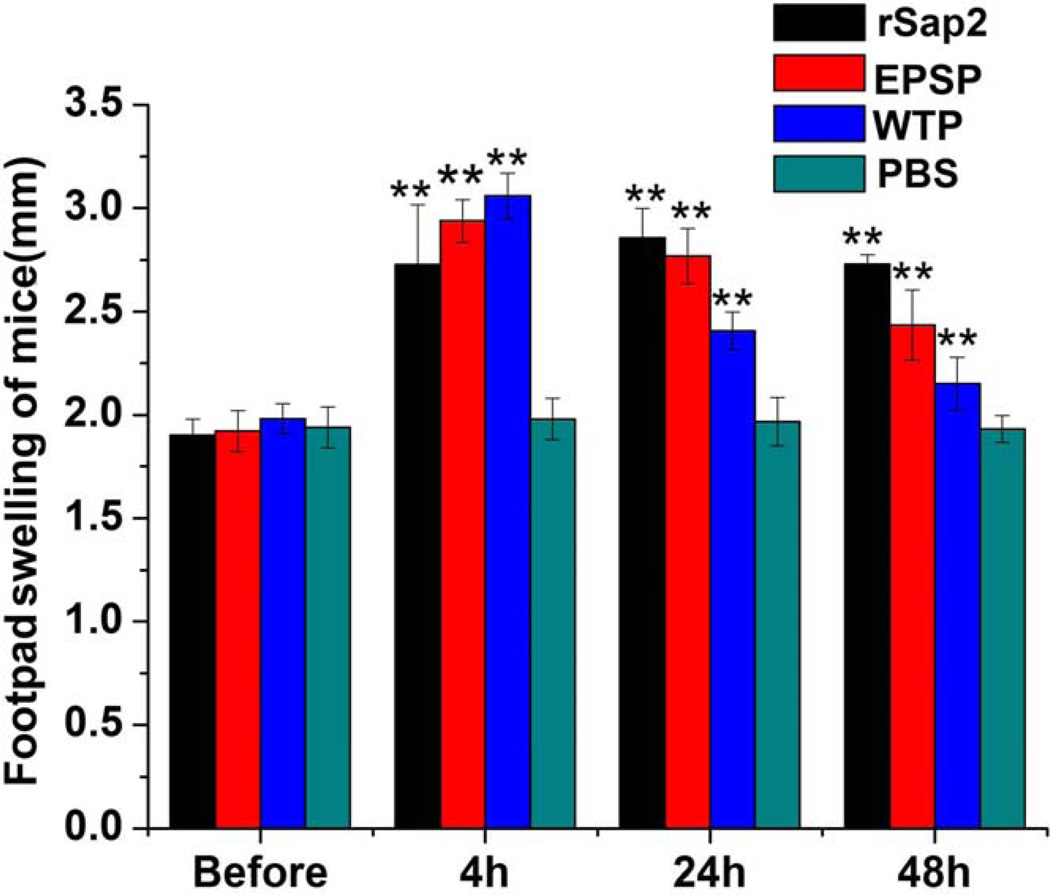
DTH reaction in the immunized mice was also induced by EPSP nanofibers. Compared with the control group in which mice were injected with PBS, mice immunized with rSap2, EPSP or WTP nanofibers showed significantly greater footpad swelling (Figure 6).
Figure 6.
DTH response of immunized mice with rSap2, EPSP, WTP or PBS. One week after the third time immunization, mice were injected with 15 µg rSap2, 15 µg EPSP, 15 µg WTP or PBS in the left footpad. It was concluded that the thickness of footpads of mice in EPSP group, rSap2 group or WTP group was significantly larger than the control group at 4 h, 24 h, and 48 h after injection. * Significant (p<0.05), ** Very significant (p<0.01).
2.4. Assessment of using EPSP to protect systemic CA infection
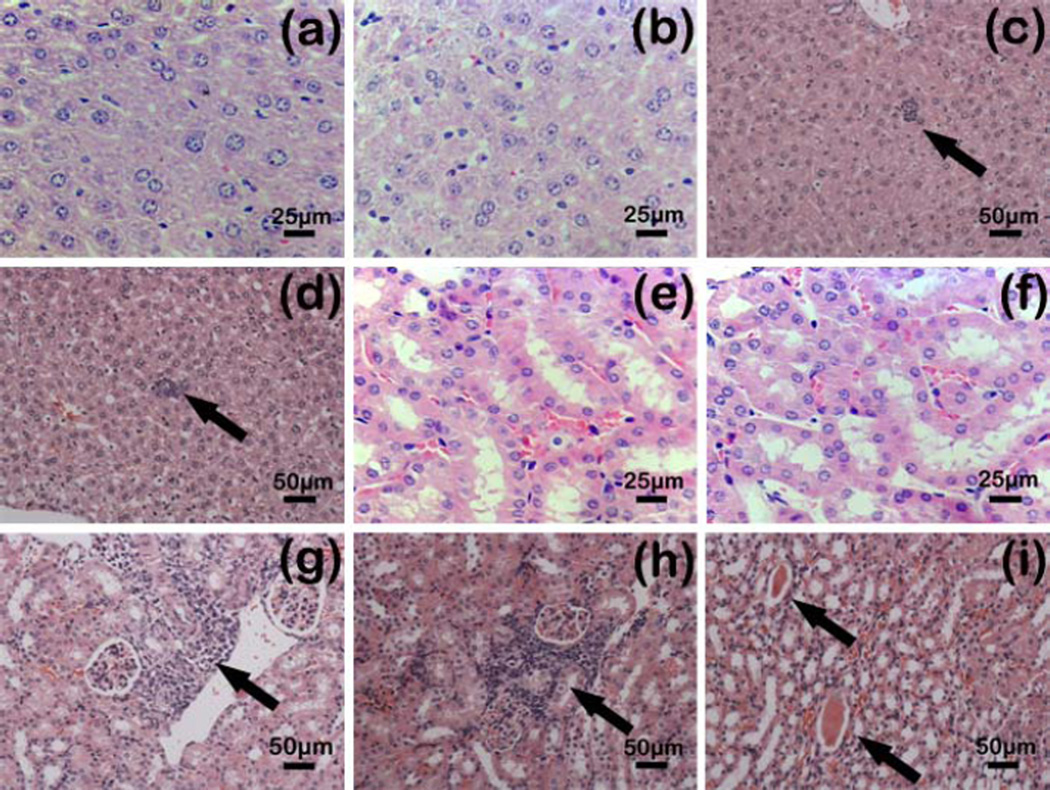
To estimate the immune protection effect of the EPSP nanofibers in the immunized mice against candidiasis, CA cells (2×l06) were injected intravenously to each mouse one week after the last immunization. CA colonies in kidneys were counted. Our results showed that the mice immunized with EPSP nanofibers significantly had fewer colony forming units (CFUs) in the kidneys than those immunized with the WTP nanofibers or injected with PBS (Table 1). In addition, there was no statistical difference between the groups immunized with EPSP nanofibers and rSap2 (Table 1). Histopathological examination (Figure 7) further proved that mice immunized with the EPSP nanofibers or rSap2 had less lesion in the kidneys and livers. On the contrary, significant protein tubes or inflammatory cell infiltration were seen in the kidneys or livers of the mice immunized with WTP or PBS (Figure 7c, d, g, f, i).
Table 1.
Colonization of CA in kidneys of immunized mice.
| Treatment groupa | CFU in Kidney (log10/g of kidney)b | pc |
|---|---|---|
| rSap2 | 2.34±0.04 | <0.05 |
| EPSP nanofibers | 2.71±0.09 | <0.05 |
| WTP nanofibers | 3.29±0.04 | NS (not significant) |
| PBS | 3.37±0.02 |
One week after the third time immunization with rSap2, EPSP, WTP or PBS, mice were inoculated with 2×106 CA cells.
Kidneys of mice were collected, weighed and homogenized with a glass grinder in sterile physiological saline. Then CFU of CA in kidneys was counted.
Statistic analysis with student’s t-test showed that immunization with EPSP (p<0.05) or rSap2 (p<0.05) significantly decreased the CFU in kidneys of mice against candidiasis compared with PBS group and WTP group.
Figure 7.
Microscopic observation of H&E-stained paraffin section of kidneys and livers of immunized mice against CA (2×106) infection. One week after the third time immunization, all mice were challenged with CA. Histological sections (3–5 mm) of kidneys and livers of each mice were stained with H&E, observed with microscope and then photographed by digital cameras. (a) Livers from mice immunized with rpSap2. Magnification: 400×, (b) Livers from mice immunized with EPSP. Magnification: 400×, (c) Livers from mice immunized with WTP. Magnification: 200×, (d) Livers from mice immunized with PBS. Magnification: 200×, (e) Kidneys from mice immunized with rpSap2. Magnification: 400×, (f) Kidneys from mice immunized with EPSP, Magnification: 400×, (g) Kidneys from mice immunized with WTP, Magnification: 200×, (h) and (i) Kidneys from mice immunized with PBS, Magnification: 200×. These images suggested that immunization with EPSP (b, f) or rSap2 (a, e) protected the mice against CA infection in visceral injury compared to PBS group (d, h, i) and WTP group (c, g). The arrows in (c), (d), (g) and (h) indicate infiltration of inflammatory cells, and those in (i) indicate protein tubes.
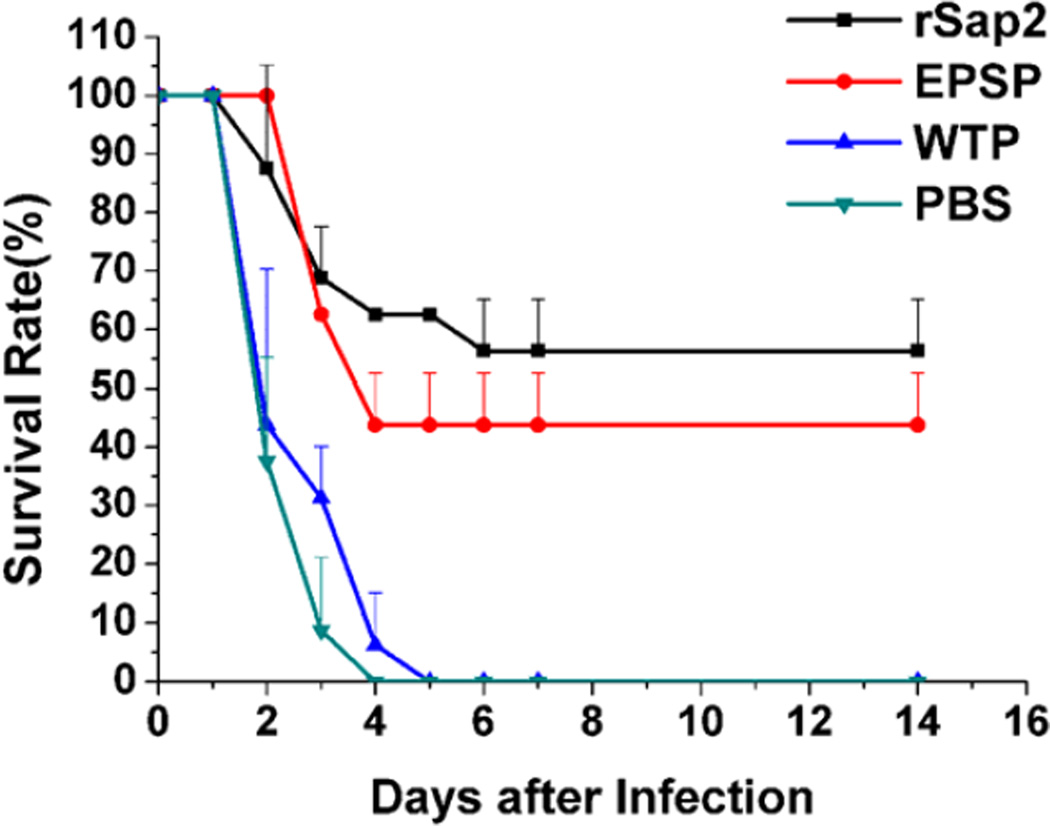
Survival rate against 107 lethal CA infection was estimated with immunized mice one week after the last immunization. The number of dead mice in each group was recorded for 14 days following challenges. Our data showed that the survival rate of the EPSP group was 43.75%, slightly lower than rSap2 group (56.25%), but significantly higher than WTP (0%) and PBS group (0%) (Figure 8).
Figure 8.
Survival percentage of immunized mice resistant to CA infection. One week after the third time immunization, each mouse was inoculated with 2×107 CA cells, and then the number of living mice was recorded for 14 days. The survival rate revealed that immunization with EPSP or rSap2 significantly prolonged the living time of mice against CA infection in comparison to PBS control and WTP group. The data is represented as mean+standard deviation.
To explore the potential function of anti-EPSP IgG against candidiasis, IgG was purified from sera collected from the immunized mice and administrated to mice infected with a lethal dose of CA cells (2×l07). It was shown that the CFUs in the kidneys of rSap2 and EPSP immunized mice were statistically less than those in the WTP immunized or PBS injected mice (Table 2).
Table 2.
CFU in kidneys of mice treated with antibody
| Treatment groupsa | CFUs in Kidneyb (log10/g of kidney) | pc |
|---|---|---|
| rSap2 | 1.30±0.46 | <0.01 |
| EPSP nanofibers | 1.34±0.54 | <0.05 |
| WTP nanofibers | 2.47±0.55 | NS (not significant) |
| PBS | 2.45±0.46 |
Mice were inoculated with 100 µL PBS containing 2×107 CA cells via an intravenous injection. Four hours later, 6 mice each group were chosen randomly for treatment with the reagents below: (1) 100 µl of purified antibody against rpSap2 at a dose of 5.0 mg/kg mice weight; (2) 100 µl of purified antibody against EPSP at a dose of 5.0 mg/kg mice weight; (3) 100 µl of purified antibody against WTP at a dose of 5.0 mg/kg mice weight; (4) 100 µl PBS at a dose of 5.0 mg/kg mice weight.
Two days after injection of antibodies, kidneys of mice were collected, weighed and homogenized with a glass grinder in sterile physiological saline. CFUs were counted and recorded.
Difference analysis with student’s t-test showed that the EPSP antibody (p<0.05) and rSap2 (p<0.01) antibody greatly decreased the CFU in kidneys of mice against candidiasis compared with the control (PBS) group.
3. Discussion
Nowadays, vaccines against fungal diseases have attracted more and more attention for the increasing incidence rates of fungal diseases worldwide. It has been shown that protein antigens can exert protective immunity against fungal diseases.[43] A protein antigen based vaccine could enhance host resistance by inducing the production of antibodies, T-cell-mediated immune response, or a combination of both.[5] In this study, the humoral and cellular immune response against candidiasis in BALB/c mice immunized with the EPSP nanofibers was tested along with the control group of rSap2.
Phage nanofibers are a powerful antigen delivery vector for two main reasons. Firstly, they could display EPS on the surface by genetic means and allow the EPS to have the similar conformation as the native protein from which the EPS is derived. Secondly, they have other advantageous characters, such as the capacity to induce the antibody response and T cell response, being non-toxic to human beings, and easy and cost-effective production.[44] In fact, because they can be mass-produced by simply infecting bacteria, they could avoid the expensive ways of producing vaccines by using animals. In this study we have constructed an EPSP where its major coat protein pⅧ was fused with EPS (Figures 1 and 2).
Sap2 is known as an important virulence factor, which is secreted by CA and functions by degrading several host proteins capable of protecting mucosal surface. Sap2 not only helps fungi to obtain necessary growth nitrogen, but also enhances the attachment, colonization, and penetration of host tissue by removing the host barriers.[45, 46] Immunization with Sap2 or truncated Sap2 could induce mice to produce anti-Sap2 antibody, and also significantly reduce the fungal burden orally and vaginally.[11, 14, 16, 47] In another study, suppressing Sap2 activity by adding the aspartyl proteinase inhibitor pepstatin A drastically reduced the cytokine response of reconstituted human vaginal epithelium (RHVE). [48] It was also found that Sap2 null mutants had a reduced potential to cause tissue damage even though other Sap members were up-regulated in these mutants.[49] Our work further showed that immunization with the rSap2 could produce antibodies in the sera, which can bind rSap2 (Figure 3). Moreover, our work demonstrated that immunization with the EPSP nanofibers could produce antibodies in the sera, which can bind rSap2 and rSap2-secreting CA (Figure 3, Figure 4). Such binding event was supposed to prevent the adhesion and migration of CA to or in the host, thereby relieving the infection.[16] Hence, collectively, our work shows that the EPSP, the nanofibers displaying an epitope from the Sap2, can have similar function as rSap2 in terms of treating CA infection as a vaccine. Namely, our work indicates that the EPS displayed on the EPSP nanofiber surface can mimic Sap2 and induce mice to produce antibodies that can bind with the Sap2-secreting CA for treating the infection. Some studies claimed that mice got significantly passive protection after inoculated with monoclonal IgM and IgG against Sap2.[16] Our results proved the similar result with anti-EPSP IgG. When the purified anti-EPSP or Sap2 monoclonal antibody was passively given to mice infected with a lethal dose of CA cells (2× l07), there was a statistically significant reduction of CFUs in the kidneys treated with either anti-EPSP antibody or rSap2 antibody compared with antibody against WTP or PBS (Table 2). The mechanism by which the anti-EPSP antibodies protect CA infection is unclear at this moment. It is likely that they decrease the adhesion and colonization of CA in host and thus relieve the CA infection in a similar manner as anti-Sap2.[14] Therefore, the protective effect of the EPSP against candidiasis proved that EPS displayed on the EPSP nanofiber surface indeed mimic the epitope of Sap2 and functioned as a vaccine together with a phage carrier.
Cellular immunity, including the CD4+ and CD8+ T cells is deemed to be critical for the elimination of fungal pathogens. CD4+ T cells come into play against fungi through secretion of T-helper (Th1 or Th17) cytokines, which can activate inflammatory cells for fungal killing and clearance and activate B cells to secrete protective antibodies.[50, 51] Recently, it was found that Th17 cells could mediate phagocyte recruitment to and activation of phagocytes at sites of infection, and IL-17 is integral for the protection against a number of fungal pathogens.[52, 53] Other studies claimed that Th17 cells, responsible for protection mediated by some vaccines against candidiasis, functioned directly, by promotion of or collaboration with, Th1 response.[52, 54–56] Observations in our study showed that the EPSP nanofibers could induce TH1 and Th17 response including the secretion of IL2, IL12, IFN-γ and IL17 in the supernatant of splenocytes (Figure 5). In addition, Th2 response with the secretion of IL10 in the experimental groups was a little stronger than that in the control group, but with no significant difference (Figure 5). This phenomenon can be explained by the reported discoveries.[57] Namely, the TH2 response is related to the subversion of host by fungi and the increased secretion of TH2 cytokines (IL-4 and IL-10) often can be seen in the progressive disease. However, neutralizing the activities of IL-4 and IL-10 may help host to restore its protective immunity.[57]
In addition to CD4+ T cells response, CD8+ T cells could be elicited with protective anti-fungal responses and resistance in the absence of obvious help from CD4+ T cells.[58, 59] In our study, stronger DTH reaction reduced by CD8+ cells was seen in the mice immunized with the EPSP nanofibers than that in the mice receiving PBS. Hence, we believe that the EPSP nanofibers elicited CD8+ immune response (Figure 6).
The reason that EPSP nanofibers could induce stronger cellular responses may be attributed to the foreign T-cell epitopes displayed on the nanofibers.[60, 61] Studies show that peptides displayed on the phages can be loaded on major histocompatibility complex (MHC) class I by a process known as cross-presentation to activate CD8+ T cells, or loaded on MHC class II by a process, which occurs through first endocytosing by antigen presenting cells (APC) and then undergoing proteolysis in the endosomal–lysosomal compartments to activate CD4+ T cells.[62, 63] These data provide further evidence showing that epitope displayed on the surface of the EPSP nanofibers can be a superior vaccine that can significantly induce cellular response.
A vaccine candidate for fungal infections usually need to satisfy one or both of these immunological themes to improve fungi killing: the ability to activate cell based, pro-inflammatory, CD4+ or CD8+ immune response of the host; the ability to induce protective antibodies in host. Observations in this study supported that immunization with the EPSP nanofibers increased the secretion of Th1 and TH17 cytokines (Figure 5), boosted the DTH response in immunized mice, and produced antibodies that can bind CA (Figs. 4 and 6). Besides, the protective immunity against candidiasis was seen in the mice immunized with the EPSP nanofibers (Figures 7–8 and Table 1). The anti-EPSP antibody can be a therapeutic cure to CA-infected mice (Table 2). Phage nanofibers are viruses that specifically infect bacteria. Thus phage nanofibers can be mass-produced in an error-free format by simply infecting bacteria, providing a cost-efficient method for the production of the nanofibers. Hence, our work implied that the EPSP nanofibers, which display a very short 5-mer peptide instead of a longer peptide[64] on the human-safe phage, might be a cost-effective and non-adjuvant effective vaccine against candidiasis.
4. Conclusion
A type of engineered viral nanofibers, EPSP nanofibers with a 5-mer EPS displayed on their side walls, are used to immunize the mice. We have characterized humoral and cellular response to the EPSP nanofibers. We found that the EPSP nanofibers could serve as an excellent subunit vaccine against candidiasis. The EPSP nanofibers not only can induce cell-based immune response in mice, but also can stimulate the mice to produce effective antibodies, which can bind CA for treating the infection, to decrease the progressive fungi loading in the kidney of mice. EPSP nanofibers can be mass-produced by infecting bacteria in a cost-efficient manner due to their nature of being the non-toxic viruses specifically infecting bacteria. In consideration of the low-cost and non-adjuvant character, they may be an effective vaccine candidate against CA infection.
5. Experimental
Reagents
CA (ATCC10231) was maintained on sabouraud’s agar at 4 °C. The pET28a–Sap2 recombinant plasmid was constructed and stored in our own laboratory. 6–8 week-old female BALB/c mice were purchased from Beijing HFK Bioscience Company. E. coli BL21 and TG1 strains were bought from Source Biosicense, USA. Freund’s incomplete and complete adjuvants were purchased from Aladdin Industrial Corporation in China.
Production and purification of rSap2 and phage nanofibers
The correct recombinant plasmid PET28a-Sap2 was transformed into E. coli BL21. After cultured in Luria-Bertani (LB) medium in the presence of 1 mM IPTG for 6 h, the E. coli bacteria were collected and decomposed by ultrasonic interaction, followed by centrifugation. After centrifugation, the protein in the precipitate was then dissolved in urea and purified with Ni affinity chromatography following the manufacturer’s instructions (GE, USA). Finally, the protein in the urea was dialyzed in PBS and quantified by spectrophotometry. The purity of rSap2 was detected by SDS-PAGE and developed with brilliant blue.
EPS was displayed on the major coat of phage nanofibers using our reported protocal.[35] EPSP was amplified by infecting E. coli TG1 strain and purified with double polyethylene glycol (PEG) precipitations. Briefly, synthesized oligonucleotides encoding EPS were inserted into the phagemid pfd88, then the resultant recombinant plasmid pfd88-EPS was transformed into E. coli TG1. After the transformed E. coli TG1 cells were cultured with the help of WTP for 6 h, EPSP was amplified and secreted into the extracellular culture. Then, the culture supernatant containing EPSP nanofibers was pooled and purified in PEG-6000/NaCl (5% PEG-6000, 0.5 M NaCl) with two consecutive precipitations. The EPSP pellet was suspended in 1 ml PBS buffer. The amount of EPSP was estimated by spectrophotometry. WTP was amplified and purified by the similar procedure. EPSP and WTP nanofibers were analyzed by SDS-PAGE with polyacrylamide gel, followed by silver staining.
Image of EPSP nanofibers
The EPSP nanofibers were characterized with atomic force microscopy (AFM) and transmission electron microscopy (TEM).
Immunization of mice
All the animal experiments were done under the international legal and institutional guidelines and approved by Institutional Animal Care and Use Committee at Northeast Normal Univeristy. Mice were divided randomly into four groups and immunized intraperitoneally for three times (on week 0, 2, and 3) with the following suspensions: (1) 25 µg rSap2 in 100 µl PBS coupled with 100 µl Freund’s complete adjuvant (FCA) for the first time as well as Freund’s incomplete adjuvant (FIA) for the second and third time; (2) 25 µg EPSP nanofibers in 200 µl PBS each time; (3) 25 µg WTP nanofibers in 200 µl PBS each time; and (4) 200 µl PBS each time.
Western blot
One week after the third immunization, the blood of the mice of all groups was collected and then the sera was seperated. Western blotting was used to assess the antibody response against rSap2 in the sera of vaccinated or negative control mice. Briefly, rSap2 protein was added to polyacrylamide gel, transferred onto nitrocellulose membrane at 75 V for 3 h, and blocked with 5% non-fat milk in TBST (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.1% Tween 20) overnight at 4 °C. The membranes were subsequently incubated with sera (1:400) for 1 h at 37 °C and then washed with TBST. After incubated with anti-mouse IgG conjugated to horseradish peroxidase (HRP) (1:2500)(ZSGB-Bio Origene, China) for 1 h at 37 °C and washed with TBST three times, the antigen-antibody complexes were detected using 3-Amino-9-ethylcarbazole (AEC) solution.
Immunofluorescence
The overnight culture of CA was incubated with fresh yeast extract peptone dextrose (YPD) medium until cultures reached logarithmic phase. 107 sporophores were seeded into 6 well tissue culture plates and cultured with 1640 medium for 4 h. After slight wash with PBS once, the cells were incubated with sera containing antibodies against EPSP or WTP for 2 h on ice. The adherent cells were fixed with 4% paraformaldehyde for 20 min and washed subsequently with PBS for 3 times. The fixed cells were permeabilized with 0.5% Triton X-100 and washed with TBST for three times. After blocking with 3% BSA at 37 °C, the cells were incubated with P-phycoerythrin (PE) conjugated goat anti-rabbit IgG (1:2000) (ZSGB-Bio Origene, China) for 45 min at 37 °C and stained with DAPI for 10 min. After washed with PBST (PBS+0.1% Tween 20) for three times, the cells were mounted and observed with a laser scanning confocal microscope.
Cytokines secretion in supernatants of the splenocytes culture
106 splenocytes from each immunized mice were dispersed in 1640 medium and seeded in 96-well plates in the presence of rSap2 (25 µg/ml), EPSP nanofibers (25 µg/ml), WTP nanofibers (25 µg/ml) or PBS individually, with each group in triplicates. Culture supernatants were collected after 48 h incubation to determine the levels of cytokines in accordance with the manufacturer’s instruction (Wuhan Boster Biological Technology, LTD. China).
Delayed-type hypersensitivity (DTH) assay
DTH assay was done one week after the final immunization. rSap2 (15 µg in 30 µl PBS), EPSP nanofibers (15 µg in 30 µl PBS), WTP nanofibers (15 µg in 30 µl PBS) or PBS (30 µl) was injected into the left footpads for each mouse. Footpad swelling was measured with a caliper at 4 h, 24 h, 48 h after the injection.
Challenge with CA strains
One week after the final immunization, three mice of each group were inoculated intravenously with 106 CA. Each group of mice was sacrificed 7 d after the infection. Then kidneys and livers were excised aseptically in two parts. One part was fixed in 10% (v/v) formalin for tissue slices. Sections (3–5 µm) of buffered paraffin-embedded tissues were stained with hematoxylin and eosin (H&E). Another part was used to assess the number of CA having migrated to the kidney and spleen. For the survival rate experiment, eight immunized mice in each group were injected intravenously with 107 CA one week after the last immunization. The number of mice that died 14 days post-infection was counted.
Passive therapy using antibodies against candidiasis
In the passive therapy assay, IgG antibody was purified firstly from the sera of all immunized mice according to the manufacturer’s instructions with HiTrap Protein G HP column (GE General Electric, USA). The purified antibodies were used to treat the mice infected by CA (107) 4 h after infection. Randomized groups of 8 mice were given one of the following antibodies intravenously: (1) 100 µl of purified antibody against rSap2 at a dose of 5.0 mg/kg weight; (2) 100 µl of purified antibody against EPSP nanofibers at a dose of 5.0 mg/kg mice weight; (3) 100 µl of purified antibody against WTP nanofibers at a dose of 5.0 mg/kg mice weight; and (4) 100 µl PBS at a dose of 5.0 mg/kg mice weight. The mice were sacrificed two days later, and yeast cell counts in kidneys were determined.
Statistical analysis
Numerical data were analyzed with Student’s T-test to determine statistical significance.
Acknowledgments
We would like to thank the grants from the National Natural Science Foundation of China (81028010 and 81373231), Jilin Provincial Government of the People's Republic of China (20130727034YY) and the Ministry of Science and Technology of the People's Republic of China (2014DFA31740). Y.Z., X.L., B.R.C. and C.B.M. would like to thank the financial support from National Institutes of Health (CA200504 and CA195607), National Science Foundation (CMMI-1234957 and CBET-1512664), Department of Defense office of the Congressionally Directed Medical Research Programs (W81XWH-15-1-0180), Oklahoma Center for Adult Stem Cell Research (434003) and Oklahoma Center for the Advancement of Science and Technology (HR14-160). M.Y. acknowledges the support of National Natural Science Foundation of China (21172194), Projects of Zhejiang Provincial Science and Technology Plans (2012C12910), Silkworm Industry Science and Technology Innovation Team (2011R50028), China Agriculture Research System (CARS-22-ZJ0402), and National High Technology Research and Development Program 863 (2013AA102507).
Contributor Information
Yanyan Huai, Institute of Cytology and Genetics, School of Life Sciences, Northeast Normal University, 5268 Renmin Street, Changchun City, Jilin Province130024, China; Department of Chemistry & Biochemistry, Stephenson Life Sciences Research Center University of Oklahoma, 101 Stephenson Parkway, Norman, OK73019-5300, USA.
Shuai Dong, Institute of Cytology and Genetics, School of Life Sciences, Northeast Normal University, 5268 Renmin Street, Changchun City, Jilin Province130024, China.
Ye Zhu, Department of Chemistry & Biochemistry, Stephenson Life Sciences Research Center University of Oklahoma, 101 Stephenson Parkway, Norman, OK73019-5300, USA.
Xin Li, Department of Chemistry & Biochemistry, Stephenson Life Sciences Research Center University of Oklahoma, 101 Stephenson Parkway, Norman, OK73019-5300, USA.
Dr. Binrui Cao, Department of Chemistry & Biochemistry, Stephenson Life Sciences Research Center University of Oklahoma, 101 Stephenson Parkway, Norman, OK73019-5300, USA
Prof. Xiang Gao, Institute of Cytology and Genetics, School of Life Sciences, Northeast Normal University, 5268 Renmin Street, Changchun City, Jilin Province130024, China
Prof. Mingying Yang, Institute of Applied Bioresource Research, College of Animal Science, Zhejiang University, Yuhangtang Road 866, Hangzhou, 310058, China
Prof. Li Wang, Institute of Cytology and Genetics, School of Life Sciences, Northeast Normal University, 5268 Renmin Street, Changchun City, Jilin Province130024, China
Prof. Chuanbin Mao, Department of Chemistry & Biochemistry, Stephenson Life Sciences Research Center University of Oklahoma, 101 Stephenson Parkway, Norman, OK73019-5300, USA School of Materials Science and Engineering, Zhejiang University, Hangzhou, Zhejiang 310027, China.
References
- 1.Mayer FL, Wilson D, Hube B. Virulence. 2013;4:119. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyes DL, Richardson JP, Naglik JR. Virulence. 2015;6:338. doi: 10.1080/21505594.2015.1012981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatic M, Stanic-Canji D, Draskovic B, Komarcevic A, Gajdobranski D. Med. Pregl. 2003;56:537. doi: 10.2298/mpns0312537t. [DOI] [PubMed] [Google Scholar]
- 4.Prasad R, Panwar SL, Smriti Adv. Microb. Physiol. 2002;46:155. doi: 10.1016/s0065-2911(02)46004-3. [DOI] [PubMed] [Google Scholar]
- 5.Cutler JE, Deepe GS, Jr, Klein BS. Nat. Rev. Microbiol. 2007;5:13. doi: 10.1038/nrmicro1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zakikhany K, Thewes S, Wilson D, Martin R, Albrecht A, Hube B. Nihon Ishinkin Gakkai Zasshi. 2008;49:245. doi: 10.3314/jjmm.49.245. [DOI] [PubMed] [Google Scholar]
- 7.Cowen LE, Singh SD, Kohler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, Lindquist S. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2818. doi: 10.1073/pnas.0813394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naglik JR, Challacombe SJ, Hube B. Microbiol. Mol. Biol. Rev. 2003;67:400. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassone A, De Bernardis F, Santoni G. Infect. Immun. 2007;75:4675. doi: 10.1128/IAI.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naglik JR, Moyes D, Makwana J, Kanzaria P, Tsichlaki E, Weindl G, Tappuni AR, Rodgers CA, Woodman AJ, Challacombe SJ, Schaller M, Hube B. Microbiology. 2008;154:3266. doi: 10.1099/mic.0.2008/022293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correia A, Lermann U, Teixeira L, Cerca F, Botelho S, da Costa RM, Sampaio P, Gartner F, Morschhauser J, Vilanova M, Pais C. Infect. Immun. 2010;78:4839. doi: 10.1128/IAI.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor BN, Hannemann H, Sehnal M, Biesemeier A, Schweizer A, Rollinghoff M, Schroppel K. Infect. Immun. 2005;73:7061. doi: 10.1128/IAI.73.10.7061-7063.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albrecht A, Felk A, Pichova I, Naglik JR, Schaller M, de Groot P, Maccallum D, Odds FC, Schafer W, Klis F, Monod M, Hube B. J. Biol. Chem. 2006;281:688. doi: 10.1074/jbc.M509297200. [DOI] [PubMed] [Google Scholar]
- 14.Vilanova M, Teixeira L, Caramalho I, Torrado E, Marques A, Madureira P, Ribeiro A, Ferreira P, Gama M, Demengeot J. Immunology. 2004;111:334. doi: 10.1111/j.1365-2567.2004.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bernardis F, Liu H, O'Mahony R, La Valle R, Bartollino S, Sandini S, Grant S, Brewis N, Tomlinson I, Basset RC, Holton J, Roitt IM, Cassone A. J. Infect. Dis. 2007;195:149. doi: 10.1086/509891. [DOI] [PubMed] [Google Scholar]
- 16.Sandini S, La Valle R, Deaglio S, Malavasi F, Cassone A, De Bernardis F. FEMS Immunol. Med. Microbiol. 2011;62:215. doi: 10.1111/j.1574-695X.2011.00802.x. [DOI] [PubMed] [Google Scholar]
- 17.De Bernardis F, Amacker M, Arancia S, Sandini S, Gremion C, Zurbriggen R, Moser C, Cassone A. Vaccine. 2012;30:4490. doi: 10.1016/j.vaccine.2012.04.069. [DOI] [PubMed] [Google Scholar]
- 18.Ghadjari A, Matthews RC, Burnie JP. FEMS Immunol. Med. Microbiol. 1997;19:115. doi: 10.1111/j.1574-695X.1997.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q, Su QP, Wang GY, Wen DZ, Zhang YH, Bao HZ, Wang L. Mycoses. 2007;50:165. doi: 10.1111/j.1439-0507.2006.01349.x. [DOI] [PubMed] [Google Scholar]
- 20.Quanping S, Yanyan H, Yicun W, Zhigang J, Yuling G, Li W. Diagn. Microbiol. Infect. Dis. 2010;68:382. doi: 10.1016/j.diagmicrobio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Cao B, George A, Mao C. Biomacromolecules. 2011;12:2193. doi: 10.1021/bm200274r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao B, Mao C. Biomacromolecules. 2009;10:555. doi: 10.1021/bm801224q. [DOI] [PubMed] [Google Scholar]
- 23.Ngweniform P, Abbineni G, Cao B, Mao C. Small. 2009;5:1963. doi: 10.1002/smll.200801902. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Wang L, Li X, Mao C. Sci. Rep. 2013;3:1242. doi: 10.1038/srep01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbineni G, Modali S, Safiejko-Mroczka B, Petrenko VA, Mao C. Mol. Pharmaceutics. 2010;7:1629. doi: 10.1021/mp100052y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao C, Wang F, Cao B. Angew. Chem., Int. Ed. 2012;51:6411. doi: 10.1002/anie.201107824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandra N, Abbineni G, Qu X, Huai Y, Wang L, Mao C. Small. 2013;9:215. doi: 10.1002/smll.201202090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandra N, Wang DD, Zhu Y, Mao C. Angew. Chem., Int. Ed. Engl. 2013;52:11278. doi: 10.1002/anie.201301113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao B, Zhu Y, Wang L, Mao C. Angew. Chem. Int. Ed. 2013;52:11750. doi: 10.1002/anie.201303854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Nimmo SL, Cao B, Mao C. Chem. Sci. 2012;3:2639. doi: 10.1039/C2SC00583B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Cao B, Mao C. Chem. Mater. 2010;22:3630. doi: 10.1021/cm902727s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He T, Abbineni G, Cao B, Mao C. Small. 2010;6:2230. doi: 10.1002/smll.201001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao C, Solis DJ, Reiss BD, Kottmann ST, Sweeney RY, Hayhurst A, Georgiou G, Iverson B, Belcher AM. Science. 2004;303:213. doi: 10.1126/science.1092740. [DOI] [PubMed] [Google Scholar]
- 34.Setyawati MI, Xie J, Leong DT. ACS Appl. Mater. Interfaces. 2014;6:910. doi: 10.1021/am404193j. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Ju Z, Cao B, Gao X, Zhu Y, Qiu P, Xu H, Pan P, Bao H, Wang L, Mao C. ACS Nano. 2015;9:4475. doi: 10.1021/acsnano.5b01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan P, Wang Y, Zhu Y, Gao X, Ju Z, Qiu P, Wang L, Mao C. Nano Res. 2015;8:3562. doi: 10.1007/s12274-015-0856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalarical Janardhanan S, Narayan S, Abbineni G, Hayhurst A, Mao C. Mol. Cancer Ther. 2010;9:2524. doi: 10.1158/1535-7163.MCT-10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Wang L, Yang M, Zhu Y, Tomsia A, Mao C. Nano Lett. 2014;14:6850. doi: 10.1021/nl504358j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Yang M, Zhu Y, Wang L, Tomsia AP, Mao C. Adv. Mater. 2014;26:4961. doi: 10.1002/adma.201400154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu H, Cao B, Zhen Z, Laxmi AA, Li D, Liu S, Mao C. Biomaterials. 2011;32:4744. doi: 10.1016/j.biomaterials.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perham RN, Terry TD, Willis AE, Greenwood J, di Marzo Veronese F, Appella E. FEMS Microbiol. Rev. 1995;17:25. doi: 10.1111/j.1574-6976.1995.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 42.Jelinek R, Terry TD, Gesell JJ, Malik P, Perham RN, Opella SJ, Mol J. Biol. 1997;266:649. doi: 10.1006/jmbi.1996.0821. [DOI] [PubMed] [Google Scholar]
- 43.Vecchiarelli A, Pericolini E, Gabrielli E, Pietrella D. Front. Microbiol. 2012;3:294. doi: 10.3389/fmicb.2012.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prisco A, De Berardinis P. Int. J. Mol. Sci. 2012;13:5179. doi: 10.3390/ijms13045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colina AR, Aumont F, Deslauriers N, Belhumeur P, de Repentigny L. Infect. Immun. 1996;64:4514. doi: 10.1128/iai.64.11.4514-4519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldman RC, Frost DJ, Capobianco JO, Kadam S, Rasmussen RR, Abad-Zapatero C. Infect. Agents Dis. 1995;4:228. [PubMed] [Google Scholar]
- 47.Rahman D, Mistry M, Thavaraj S, Challacombe SJ, Naglik JR. Microbes Infect. 2007;9:615. doi: 10.1016/j.micinf.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaller M, Bein M, Korting HC, Baur S, Hamm G, Monod M, Beinhauer S, Hube B. Infect. Immun. 2003;71:3227. doi: 10.1128/IAI.71.6.3227-3234.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaller M, Korting HC, Borelli C, Hamm G, Hube B. Infect. Immun. 2005;73:2758. doi: 10.1128/IAI.73.5.2758-2765.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romani L. Nat. Rev. Immunol. 2011;11:275. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 51.Wuthrich M, Deepe GS, Jr, Klein B. Annu. Rev. Immunol. 2012;30:115. doi: 10.1146/annurev-immunol-020711-074958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE, Jr, Spellberg B. PLoS Pathog. 2009;5:703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nanjappa SG, Klein BS. Curr. Opin. Immunol. 2014;28:27. doi: 10.1016/j.coi.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B. J. Clin. Invest. 2011;121:554. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hung CY, Gonzalez A, Wuthrich M, Klein BS, Cole GT. Infect. Immun. 2011;79:4511. doi: 10.1128/IAI.05726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz-Arevalo D, Bagramyan K, Hong TB, Ito JI, Kalkum M. Infect. Immun. 2011;79:2257. doi: 10.1128/IAI.01311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romani L. Nat. Rev. Immunol. 2004;4:1. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 58.Fierer J, Waters C, Walls L. J. Infect. Dis. 2006;193:1323. doi: 10.1086/502972. [DOI] [PubMed] [Google Scholar]
- 59.Chiarella AP, Arruda C, Pina A, Costa TA, Ferreira RC, Calich VL. Microbes Infect. 2007;9:1078. doi: 10.1016/j.micinf.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 60.De Berardinis P, Sartorius R, Fanutti C, Perham RN, Del Pozzo G, Guardiola J. Nat. Biotechnol. 2000;18:873. doi: 10.1038/78490. [DOI] [PubMed] [Google Scholar]
- 61.Sartorius R, Pisu P, D'Apice L, Pizzella L, Romano C, Cortese G, Giorgini A, Santoni A, Velotti F, De Berardinis P. J. Immunol. 2008;180:3719. doi: 10.4049/jimmunol.180.6.3719. [DOI] [PubMed] [Google Scholar]
- 62.Gaubin M, Fanutti C, Mishal Z, Durrbach A, De Berardinis P, Sartorius R, Del Pozzo G, Guardiola J, Perham RN, Piatier-Tonneau D. DNA Cell Biol. 2003;22:11. doi: 10.1089/104454903321112451. [DOI] [PubMed] [Google Scholar]
- 63.De Berardinis P, D'Apice L, Prisco A, Ombra MN, Barba P, Del Pozzo G, Petukhov S, Malik P, Perham RN, Guardiola J. Vaccine. 1999;17:1434. doi: 10.1016/s0264-410x(98)00377-6. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Su Q, Dong S, Shi H, Gao X, Wang L. Hum Vaccin Immunother. 2014;10:1057–1063. doi: 10.4161/hv.27714. [DOI] [PMC free article] [PubMed] [Google Scholar]