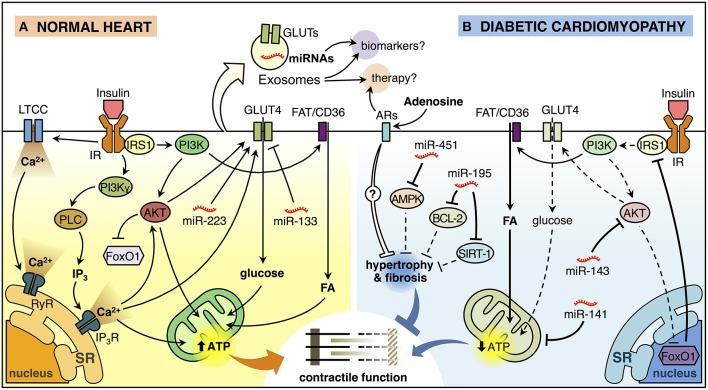
Figure 1.
Insulin signaling in the normal heart and diabetic cardiomyopathy. (A) Insulin binding to its receptor (insulin receptor, IR) promotes a biphasic [Ca2+]i response in cardiomyocytes. The first phase of insulin-dependent [Ca2+]i increase involves extracellular Ca2+ influx through L-type calcium channel (LTCC) and activation of ryanodine receptor (RyR) Ca2+ channel which in turn releases Ca2+ from the sarcoendoplasmic reticulum (SR). The second phase involves a non-inhibitory G protein coupled receptor (Gβγ subunits) that activates downstream effectors, including phosphatidylinositol 3-kinase (PI3K)γ and phospholipase C (PLC). PLC generates inositol-1,4,5-trisphosphate (IP3) that opens the IP3-ligated Ca2+channels in the SR. These mechanisms trigger the translocation of glucose transporter 4 (GLUT4) from an intracellular store to the plasma membrane (PM) and increase glucose uptake. Insulin also induces the translocation of the fatty acid (FA) transporter FAT/CD36 to the PM trough PI3K activation promoting the FA uptake. Increased lipid and glucose uptake increase mitochondrial oxidative metabolism generating adenosine triphosphate (ATP) that supports myocardial contractile function. Insulin activation of AKT, downstream of PI3K, inactivates the transcription factor FoxO1. In addition, miR-223 stimulates and miR-133 reduces GLUT4-dependent glucose uptake. (B) Insulin signaling is deficient in diabetic cardiomyopathy (DCM). FoxO1-dependent downregulation of insulin receptor substrate 1 (IRS1) and AKT are associated with reduced insulin-induced GLUT4 translocation to the PM and lower glucose uptake. At the same time, cardiomyocytes accumulate lipids, reducing mitochondrial oxidative metabolism and promoting mitochondrial uncoupling, which in turn, affects cardiomyocyte function. Also, miR-143 inhibits insulin signaling, whereas miR-141 may impact mitochondrial function and the production of ATP. On the other hand, miR-451 is associated with inhibition of AMPK, while miR-195 inhibits the expression of BCL-2 and SIRT-1. Both of these miRNAs are associated with hypertrophy, which will consequently generate contractile dysfunction. Adenosine may have a paracrine effect on cardiomyocytes, which could reduce hypertrophy and myocardial fibrosis through the activation of adenosine receptors (ARs). Exosomes can ferry miRNAs and proteins such as GLUT4 from one cell to another and therefore, they can be used as potential biomarkers or therapeutic agents/targets for DCM.