Abstract
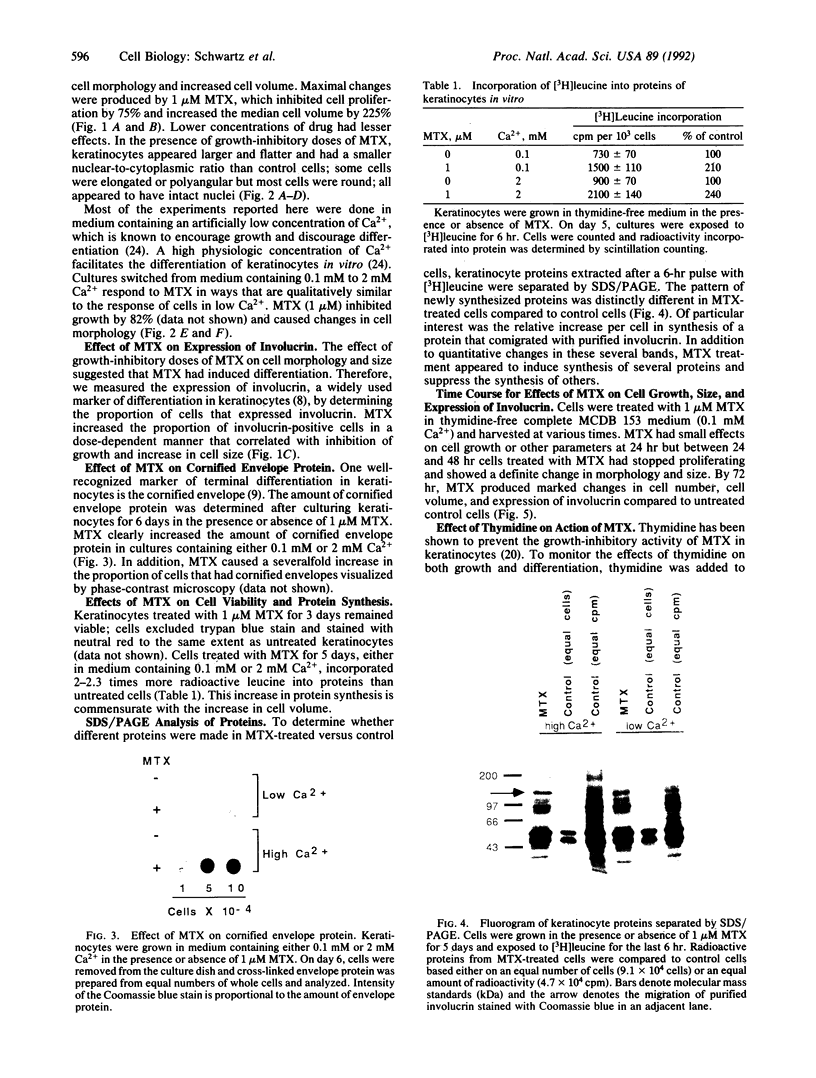
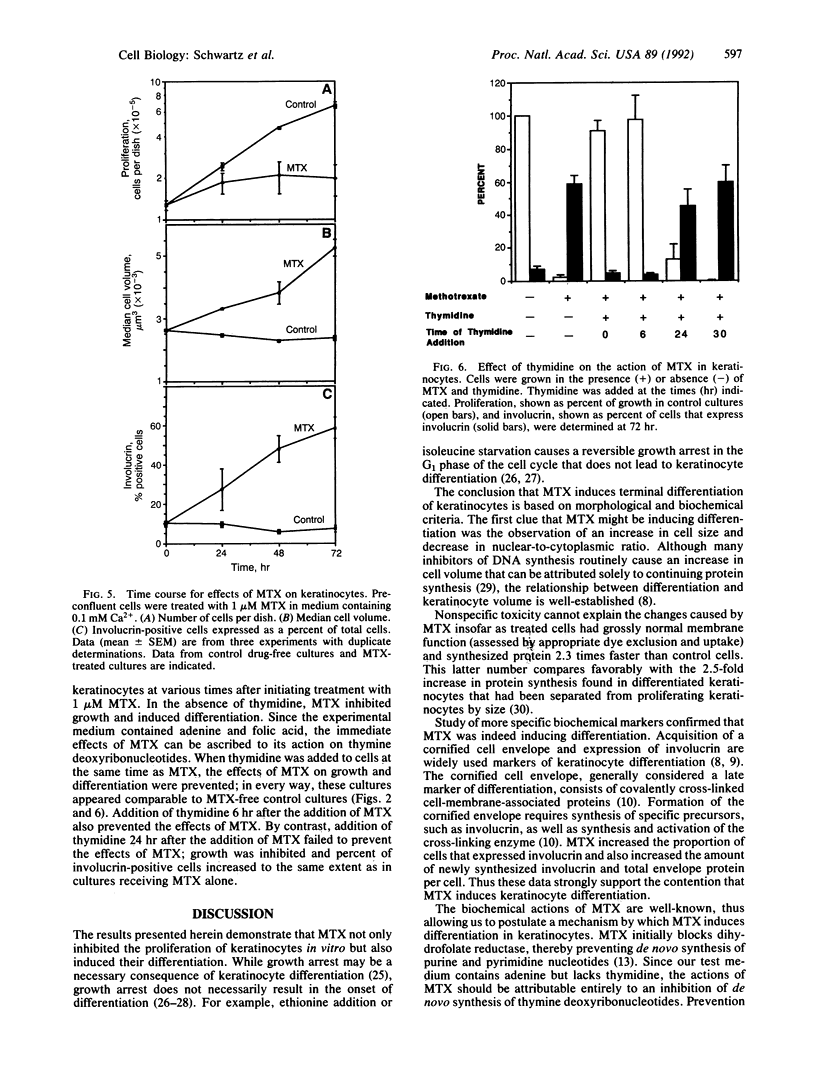
Terminal differentiation is a key element in the maintenance of tissue homeostasis in the epidermis. We show here that methotrexate (MTX) induces differentiation of human epidermal keratinocytes in vitro. MTX inhibits proliferation of keratinocytes and also induces several markers of differentiation: a change in cell morphology, a marked increase in cell size, an increase in the proportion of cells that express involucrin, and an increase in the amount of cornified envelope protein. These effects of MTX are dose- and exposure-time-dependent and become irreversible after 24 hr, approximately one population doubling time. These effects of MTX cannot be attributed to cytotoxicity since keratinocytes not only remain viable but also actively synthesize proteins. MTX causes reproducible changes in the SDS/PAGE profiles of newly synthesized proteins and, in particular, increases the amount of involucrin synthesis. Thymidine completely prevents these effects of MTX, suggesting that they are caused by a depletion of thymine deoxyribonucleotides. The effect of MTX on keratinocytes may provide a model for studying the relationship between deoxyribonucleotide metabolism and differentiation in normal cells. In addition, the ability of MTX to induce differentiation in keratinocytes suggests a mechanism to explain its therapeutic action in psoriasis.
Full text
PDF
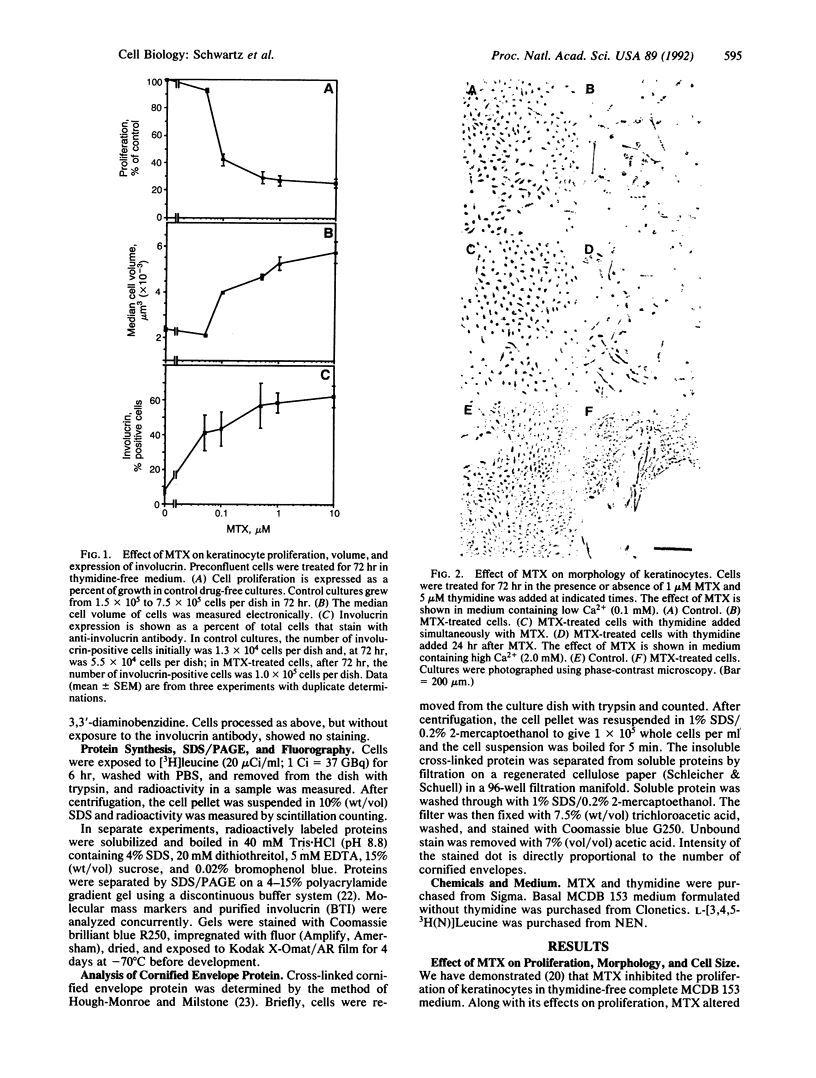

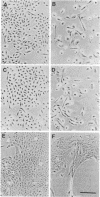
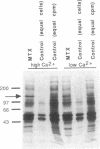
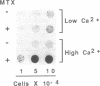
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers K. M., Taichman L. B. Kinetics of withdrawal from the cell cycle in cultured human epidermal keratinocytes. J Invest Dermatol. 1984 Feb;82(2):161–164. doi: 10.1111/1523-1747.ep12259726. [DOI] [PubMed] [Google Scholar]
- Bodner A. J., Ting R. C., Gallo R. C. Induction of differentiation of human promyelocytic leukemia cells (HL-60) by nucleosides and methotrexate. J Natl Cancer Inst. 1981 Nov;67(5):1025–1030. [PubMed] [Google Scholar]
- Boyce S. T., Ham R. G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983 Jul;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Burres N. S., Cass C. E. Inhibition of methotrexate-induced differentiation of cultured human choriocarcinoma (BeWo) cells by thymidine. Cancer Res. 1987 Oct 1;47(19):5059–5064. [PubMed] [Google Scholar]
- Dale B. A., Gown A. M., Fleckman P., Kimball J. R., Resing K. A. Characterization of two monoclonal antibodies to human epidermal keratohyalin: reactivity with filaggrin and related proteins. J Invest Dermatol. 1987 Mar;88(3):306–313. doi: 10.1111/1523-1747.ep12466185. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990 Dec;111(6 Pt 2):2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfant S. The cell cycle in psoriasis: a reappraisal. Br J Dermatol. 1976 Dec;95(6):577–590. doi: 10.1111/j.1365-2133.1976.tb07028.x. [DOI] [PubMed] [Google Scholar]
- Green H. Terminal differentiation of cultured human epidermal cells. Cell. 1977 Jun;11(2):405–416. doi: 10.1016/0092-8674(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Hohl D. Cornified cell envelope. Dermatologica. 1990;180(4):201–211. doi: 10.1159/000248031. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Pearson C. K., Fraser D. C., Prise K. M., Wong S. Y. Methotrexate-induced morphological changes mimic those seen after heat shock. J Cell Sci. 1989 Jan;92(Pt 1):37–49. doi: 10.1242/jcs.92.1.37. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meuth M. The molecular basis of mutations induced by deoxyribonucleoside triphosphate pool imbalances in mammalian cells. Exp Cell Res. 1989 Apr;181(2):305–316. doi: 10.1016/0014-4827(89)90090-6. [DOI] [PubMed] [Google Scholar]
- Milstone L. M. Population dynamics in cultures of stratified squamous epithelia. J Invest Dermatol. 1983 Jul;81(1 Suppl):69s–74s. doi: 10.1111/1523-1747.ep12540629. [DOI] [PubMed] [Google Scholar]
- Newburger A. E., Weinstein G. D., McCullough J. L. Biological and biochemical actions of methotrexate in psoriasis. J Invest Dermatol. 1978 Apr;70(4):183–186. doi: 10.1111/1523-1747.ep12541301. [DOI] [PubMed] [Google Scholar]
- Newton J. A., Camplejohn R. S., Bowyer C., McGibbon D. H., Wright N. A. Study of psoriatic epidermal cell kinetics and cell death after oral methotrexate. Dermatologica. 1985;171(6):469–473. doi: 10.1159/000249475. [DOI] [PubMed] [Google Scholar]
- Pittelkow M. R., Wille J. J., Jr, Scott R. E. Two functionally distinct classes of growth arrest states in human prokeratinocytes that regulate clonogenic potential. J Invest Dermatol. 1986 Apr;86(4):410–417. doi: 10.1111/1523-1747.ep12285684. [DOI] [PubMed] [Google Scholar]
- Reichard P. Regulation of deoxyribotide synthesis. Biochemistry. 1987 Jun 16;26(12):3245–3248. doi: 10.1021/bi00386a001. [DOI] [PubMed] [Google Scholar]
- Reiss M., Gamba-Vitalo C., Sartorelli A. C. Induction of tumor cell differentiation as a therapeutic approach: preclinical models for hematopoietic and solid neoplasms. Cancer Treat Rep. 1986 Jan;70(1):201–218. [PubMed] [Google Scholar]
- Ross D. W. Cell volume growth after cell cycle block with chemotherapeutic agents. Cell Tissue Kinet. 1976 Jul;9(4):379–387. doi: 10.1111/j.1365-2184.1976.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Schwartz P. M., Milstone L. M. Dipyridamole potentiates the growth-inhibitory action of methotrexate and 5-fluorouracil in human keratinocytes in vitro. J Invest Dermatol. 1989 Oct;93(4):523–527. doi: 10.1111/1523-1747.ep12284073. [DOI] [PubMed] [Google Scholar]
- Sokoloski J. A., Beardsley G. P., Sartorelli A. C. Mechanism of the induction of the differentiation of HL-60 leukemia cells by antifolates. Cancer Commun. 1989;1(3):199–207. [PubMed] [Google Scholar]
- Taichman L. B., Prokop C. A. Synthesis of keratin proteins during maturation of cultured human keratinocytes. J Invest Dermatol. 1982 Jun;78(6):464–467. doi: 10.1111/1523-1747.ep12510153. [DOI] [PubMed] [Google Scholar]
- Taylor I. W., Tattersall M. H. Methotrexate cytotoxicity in cultured human leukemic cells studied by flow cytometry. Cancer Res. 1981 Apr;41(4):1549–1558. [PubMed] [Google Scholar]
- WERKHEISER W. C. THE BIOCHEMICAL, CELLULAR, AND PHARMACOLOGICAL ACTION AND EFFECTS OF THE FOLIC ACID ANTAGONISTS. Cancer Res. 1963 Sep;23:1277–1285. [PubMed] [Google Scholar]
- Watt F. M., Green H. Involucrin synthesis is correlated with cell size in human epidermal cultures. J Cell Biol. 1981 Sep;90(3):738–742. doi: 10.1083/jcb.90.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M., Jordan P. W., O'Neill C. H. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein G. D., Goldfaden G., Frost P. Methotrexate. Mechanism of action on DNA synthesis in psoriasis. Arch Dermatol. 1971 Sep;104(3):236–243. doi: 10.1001/archderm.104.3.236. [DOI] [PubMed] [Google Scholar]
- Weinstein G. D., Jeffes E., McCullough J. L. Cytotoxic and immunologic effects of methotrexate in psoriasis. J Invest Dermatol. 1990 Nov;95(5 Suppl):49S–52S. doi: 10.1111/1523-1747.ep12505777. [DOI] [PubMed] [Google Scholar]
- Weinstein G. D., McCullough J. L., Ross P. A. Cell kinetic basis for pathophysiology of psoriasis. J Invest Dermatol. 1985 Dec;85(6):579–583. doi: 10.1111/1523-1747.ep12283594. [DOI] [PubMed] [Google Scholar]
- Wilke M. S., Hsu B. M., Wille J. J., Jr, Pittelkow M. R., Scott R. E. Biologic mechanisms for the regulation of normal human keratinocyte proliferation and differentiation. Am J Pathol. 1988 Apr;131(1):171–181. [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Kilkenny A. E., Steinert P. M., Roop D. R. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989 Sep;109(3):1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]