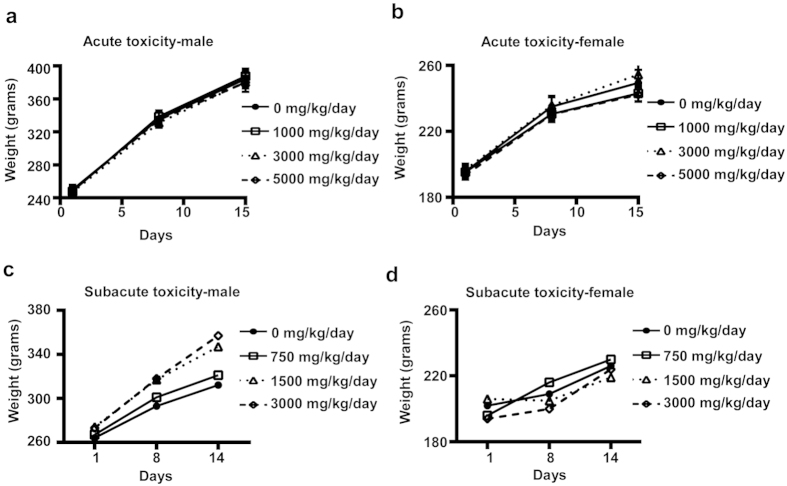
Figure 2. Short-term acute and subacute oral toxicity for the Spirulina extract.
(a) 14-day acute oral toxicity study was conducted in Sprague-Dawley rats given 0, 1,000, 3,000, or 5,000 mg/kg of Spirulina extract. Body weight changes were tracked for (a) male and (b) female rats during the study period, and no significant changes were observed. (a) 14-day subacute study, in which Sprague-Dawley rats were subjected to repeated dosing with 750, 1,500, or 3,000 mg/kg/day of Spirulina extract for 14 consecutive days, was also conducted. Body weight changes for (c) male and (d) female rats were measured on Day 1, Day 8, and Day 14. All data are presented as mean ± SD (N = 10).