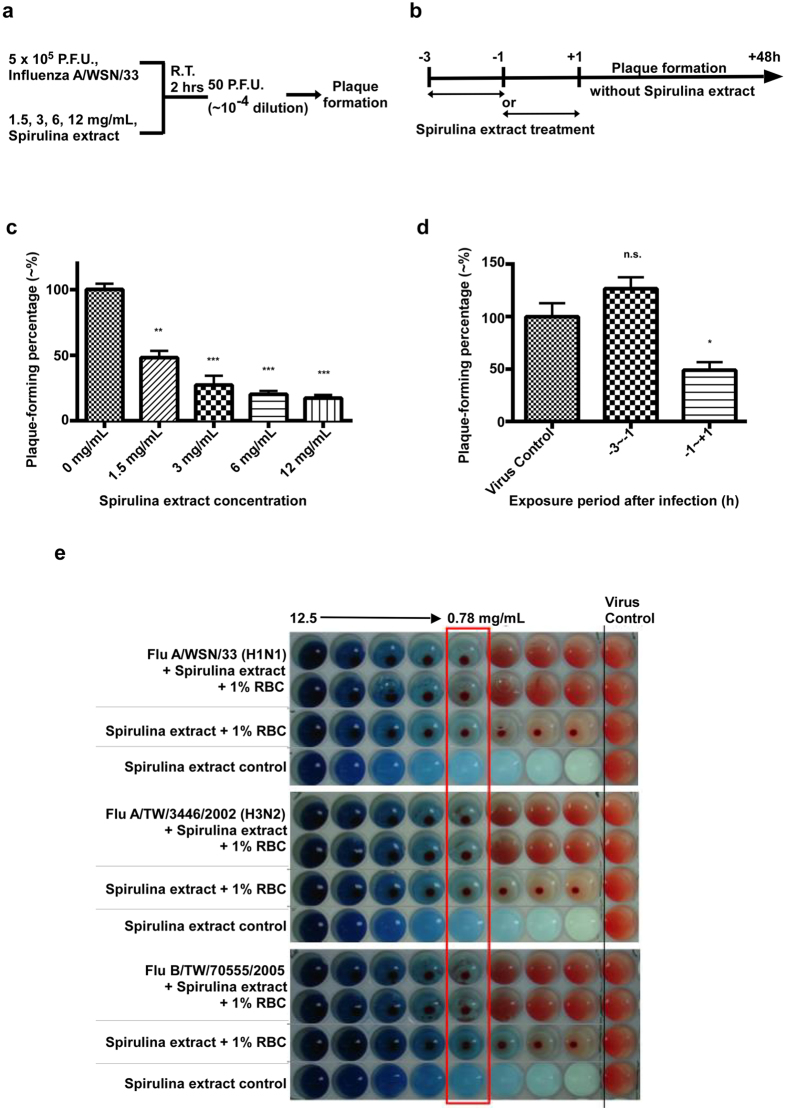
Figure 4. Spirulina extract inhibits influenza virus infection by disrupting hemagglutination.
(a) 5 × 105 PFUs of the influenza A/WSN/33(H1N1) virus was incubated with 1.5, 3, 6, or 12 mg/mL of Spirulina extract at room temperature for 2 hours, after which cultures underwent a 104-fold dilution to derive samples containing ~50 PFU and negligible amounts of Spirulina extract, which were then used in a plaque assay to assess viral viability. (b) Plaque assays showed that plaque formation percentages for Spirulina extract-treated viruses were significantly lower than untreated controls. Statistical analysis was performed with the two-tailed unpaired t-test, and used as the basis to label results as ns, not significant; *P ≤ 0.05; **P < 0.01; ***P < 0.001. (c) Monolayer MDCK cells in 6-well plates were either treated with 3 mg/mL of Spirulina extract for 2 hours, after which the culture medium was replaced with fresh medium containing no Spirulina extract (the −3 ~ −1 group), or treated with 3 mg/mL of Spirulina extract just prior to and during viral adsorption (the −1 ~ +1 group). 50 P.F.U. of the influenza A/WSN/33(H1N1) virus were added to cultures at Hour 0, and a plaque assay was conducted to assess viral viability after 48 hours of incubation. (d) Plaque assays showed that the plaque formation percentage of the −3 ~ −1 group was comparable to the untreated virus control, while plaque formation was significantly inhibited in the −1 ~ +1 group. Statistical analysis was performed with the two-tailed unpaired t-test, and used as the basis to label results as ns, not significant; *P ≤ 0.05; **P ≤ 0.01; or ***P ≤ 0.001 (e) Varying concentrations of Spirulina extract were added to 96-well plates containing 1% of guinea pig RBCs and 4 HA units of influenza A/WSN/33(H1N1), A/TW/3446/02(H3N2), or B/TW/70555/05 viruses. In the virus control column at far right, no Spirulina extract was added. Results showed that concentrations of Spirulina extract above 0.78 mg/mL were capable of inhibiting influenza hemagglutination.