Table 3.
Substrate Scope of Pt-Catalyzed C-H Hydroxylation of Secondary and Tertiary Aminesa
| entry | major product | isolated yield | isolated selectivity (crude selectivity) |
|---|---|---|---|
| 1 | 87% | >20 : 1 (8 : 1) |
|
| 2 | 76% | 7 : 1 (5 : 1) |
|
| 3 | 47% | >10 : 1 (3 : 1) |
|
| 4 | 54% | >20 : 1 (8 : 1) |
|
| 5 | 46% | >20 : 1 (>20 : 1) |
|
| 6b | 65% | >10 : 1 (5 : 1) |
|
| 7c | 88% | >20 : 1 (>20 : 1) |
|
| 8d | 102% | >20 : 1 (7 : 1) |
|
| 9e | 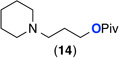 |
90% | >20 : 1 (14 : 1) |
| 10e | 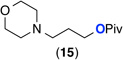 |
122% | >20 : 1 (10 : 1) |
| 11f | 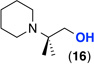 |
<5%g | ---- |
| 12f | 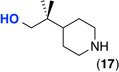 |
<5%g | ---- |
General conditions: 1 mol % K2PtCl4, 1 equiv CuCl2, 5 equiv of amine, 5.5 equiv H2SO4 (1.1 equiv relative to amine), 150 °C, 24 h.
Entry 6: Amine used as HCl salt, 10 mol % K2PtCl4.
Entry 7: 15 equiv amine, 16.5 equiv H2SO4, 48 h.
Entry 8: 0.5 mol % K2PtCl4, 15 equiv amine, 16.5 equiv H2SO4.
Entries 9–11: 5 mol % K2PtCl4.
Entries 11–12: amine used as HCl salt, 10 mol % K2PtCl4;
Yields estimated by 1H NMR analysis of crude reaction mixtures; <5% of C(sp3)–H hydroxylation or C(sp3)–H chlorination products were observed.
