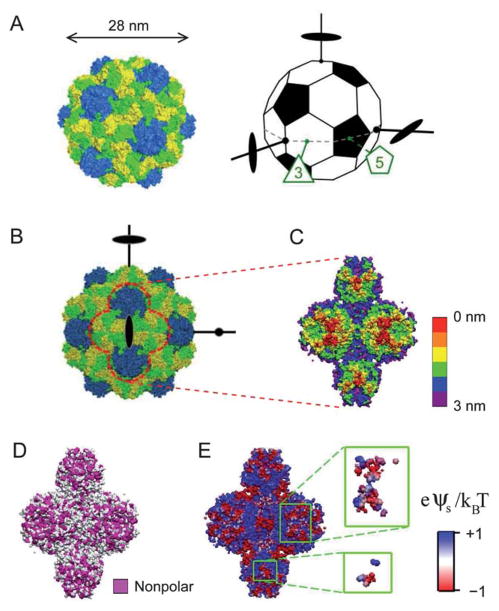
Fig. 1.
(A) The capsid of TYMV (left) consists of 180 copies of a protein subunit, with 60 subunits forming 12 pentamers (blue) and 120 subunits forming 20 hexamers (yellow and green). The truncated icosahedron (right) illustrates the virus orientation. A set of 3 orthogonal 2-fold axes, an equatorial 3-fold axis, and an equatorial 5-fold axis are indicated. (B) Projection of TYMV along a 2-fold axis. (C)–(E) The 2-fold axis views of TYMV’s exterior surface, corresponding to 2 hexagonal and 2 pentagonal knobs: (C) depth profile, (D) nonpolar residue distribution, and (E) electrostatic surface potential.18 Insets in (E) represent frontmost surfaces with depth ≤0.5 nm.