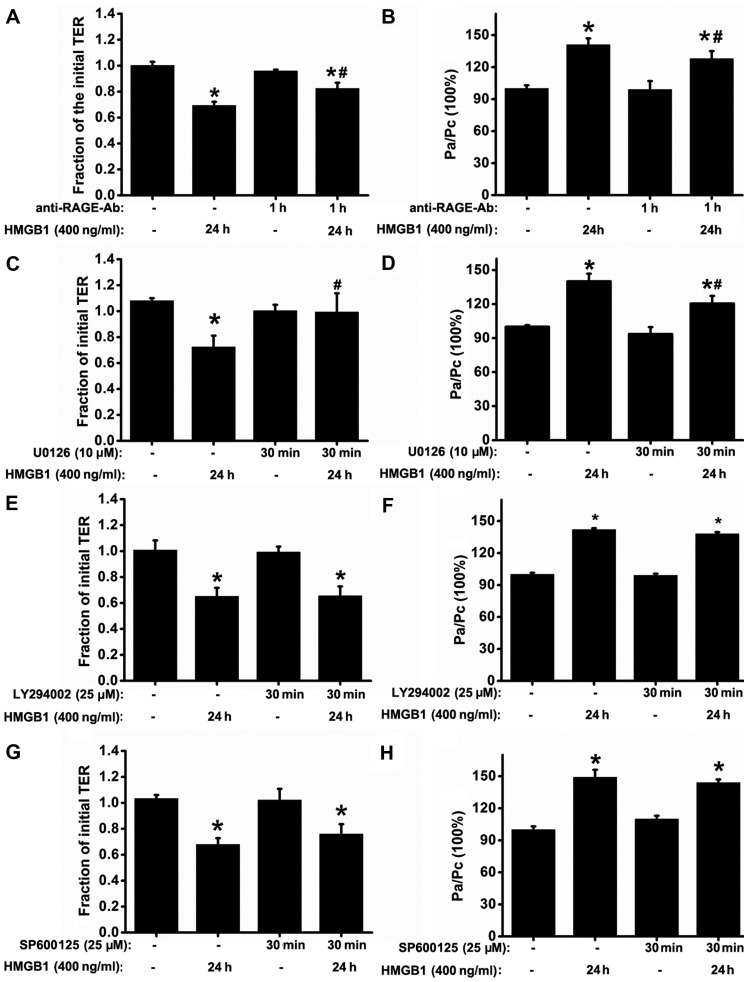
Figure 4.
Blockade of the receptor for advanced glycation end-products (RAGE)/extracellular signal-regulated kinase (ERK)1/2 pathway attenuates high-mobility group box 1 protein (HMGB1)-induced epithelial barrier dysfunction in 16HBE cells. (A and B) To ascertain that the barrier dysfunction was mediated by RAGE, 16HBE cells were treated with or without anti-RAGE antibody (5 µg/ml) for 1 h prior to stimulation with HMGB1 for 24 h (n=6 experiments) and then the transepithelial electrical resistance (TER) and FITC-dextran permeability were measured. To determine that barrier dysfunction depended on the activation of mitogen-activated protein kinases (MAPKs) or phosphatidylinositol 3-kinase (PI3K)/Akt signaling, TER and FITC-dextran permeability were measured (n=6 experiments) after the 16HBE cells were treated with or without the related signaling inhibitors: [(C and D) U0126 (ERK inhibitor, 10 µM), (E and F) LY294002 (PI3K/Akt inhibitor, 25 µM)], and (G and H) SP600125 (JNK inhibitor, 25 µM)], prior to stimulatoin with HMGB1 for 24 h. The levels of TER were normalized to those prior to HMGB1 or medium only (control) stimulation, and the levels of the FITC-dextran permeability were normalized to the ratio between HMGB1 and medium only (control) stimulation, which was denoted by Pa/Pc. P-values were calculated using the ANOVA test. *P<0.05 vs. control; #P<0.05 vs. stimulation with 400 ng/ml HMGB1.