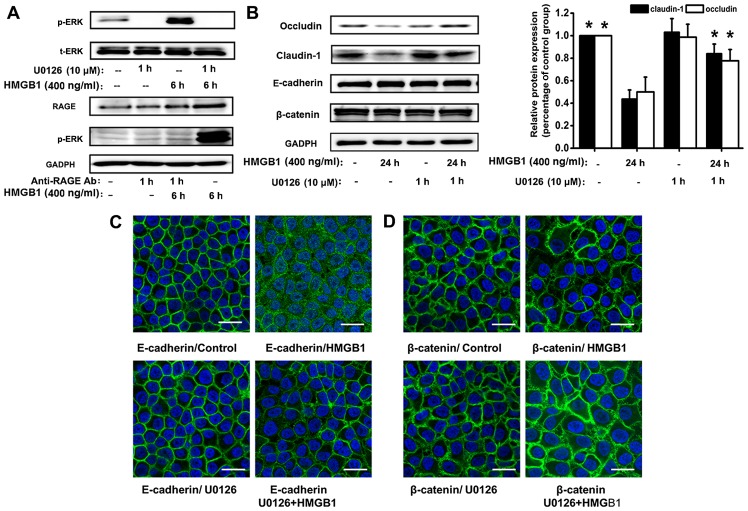
Figure 5.
Activation of the receptor for advanced glycation end-products (RAGE)/extracellular signal-regulated kinase (ERK)1/2 pathway by high-mobility group box 1 protein (HMGB1) is involved in the disruption of the epithelial intercellular contacts. The 16HBE cells were exposed to 400 ng/ml HMGB1 for the indicated periods of time. (A) 16HBE cells were treated with or without U0126 (ERK inhibitor, 10 µM), prior to stimulation with HMGB1 for 6 h, phosphorylated (p-)ERK, and the total (t-)ERK were detected by western blot analysis. To confirm the association between RAGE and the activation of the ERK1/2 pathway, the 16HBE cells were treated with or without anti-RAGE antibody (5 µg/ml) for 1 h prior to stimulation with HMGB1 for 6 h. The anti-RAGE antibody suppressed the HMGB1-induced p-ERK1/2; the response was verified by western blot analysis. (B) E-cadherin, β-catenin, occludin and claudin-1 were detected using western blot analysis. U0126 inhibited the HMGB1-mediated downregulation of the tight junction proteins, occludin and claudin-1. The ERK inhibitor, U0126, prevented the delocalization of (C) E-cadherin and (D β-catenin in response to HMGB1, verified by immunofluorescence staining. Representatives of 3 independent experiments are shown. *P<0.05 vs. HMGB1-stimulated group; scale bar, 50 mm.