Abstract
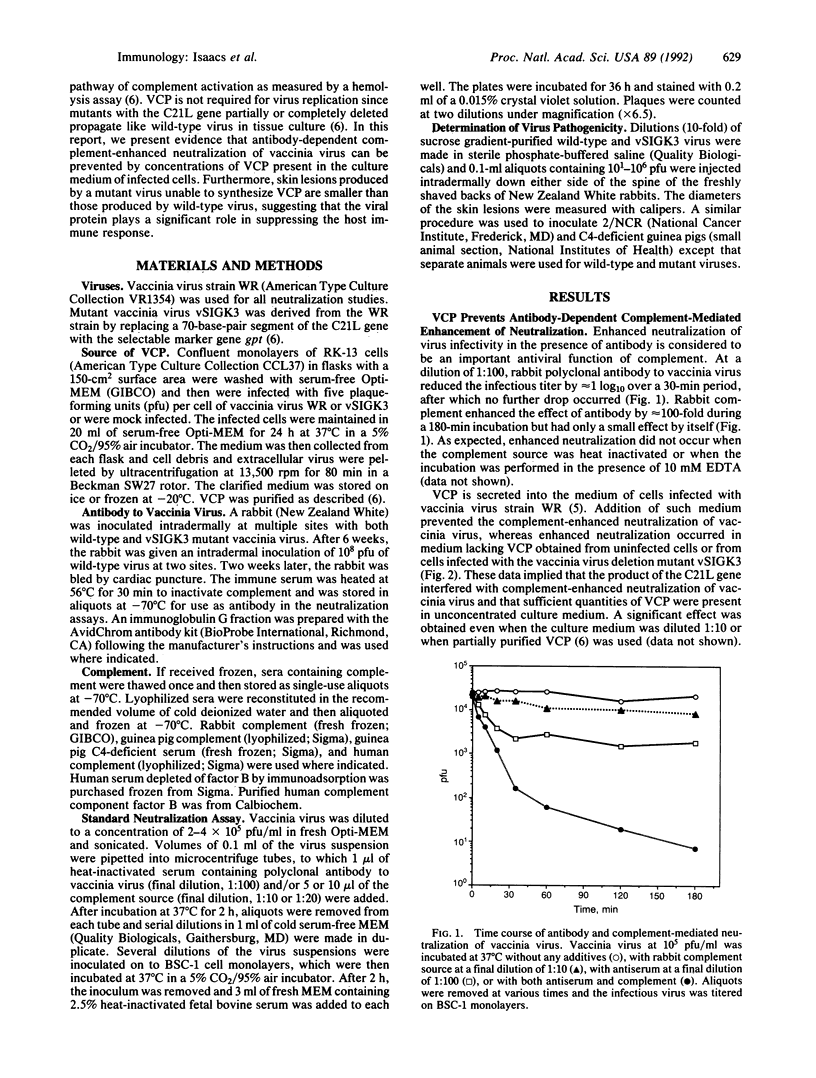
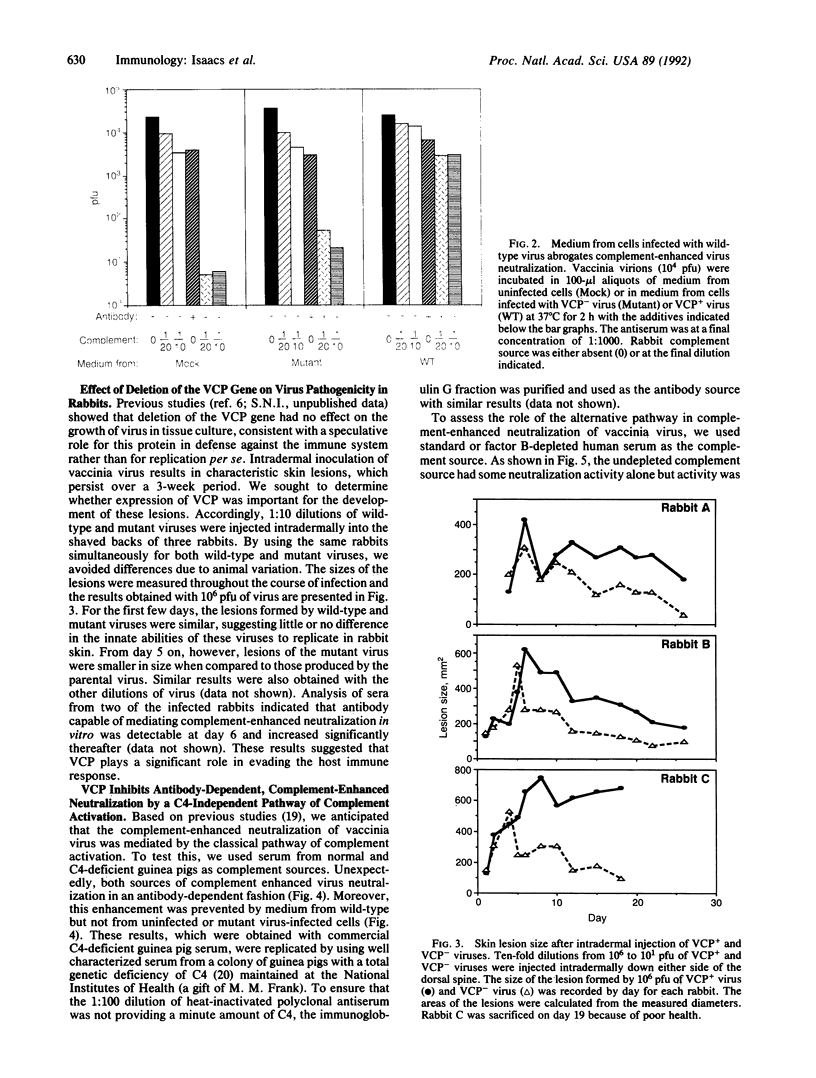
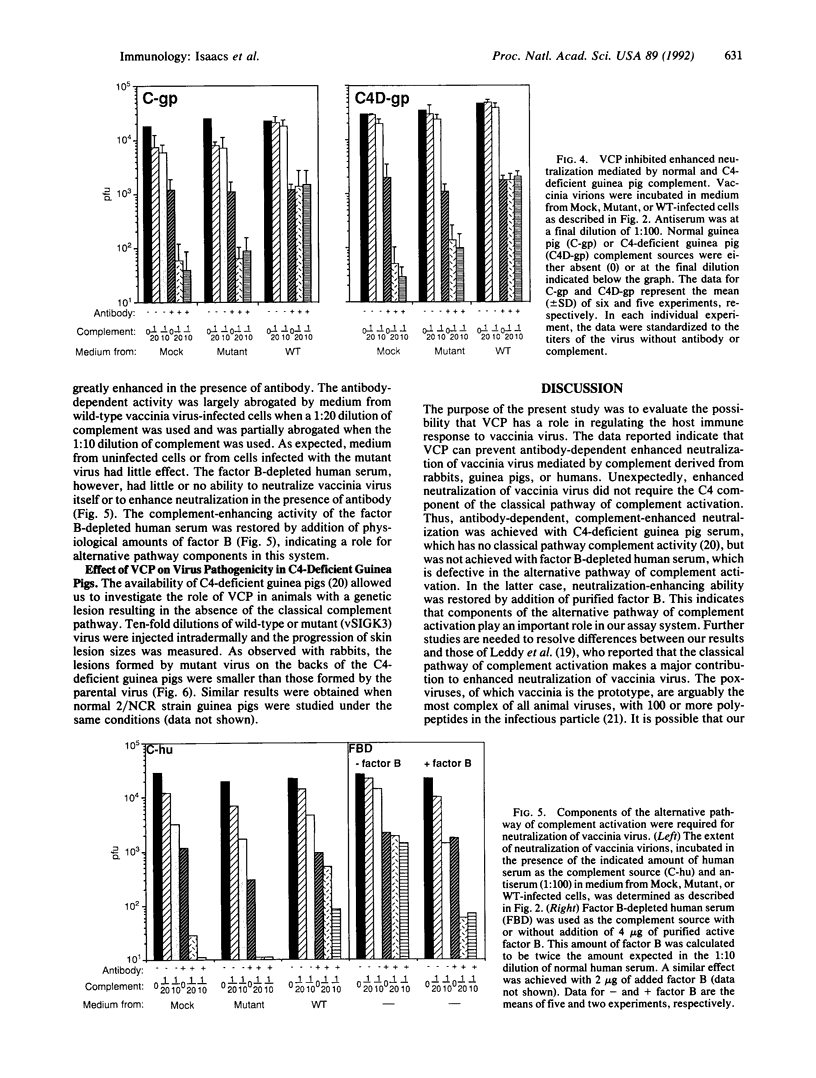
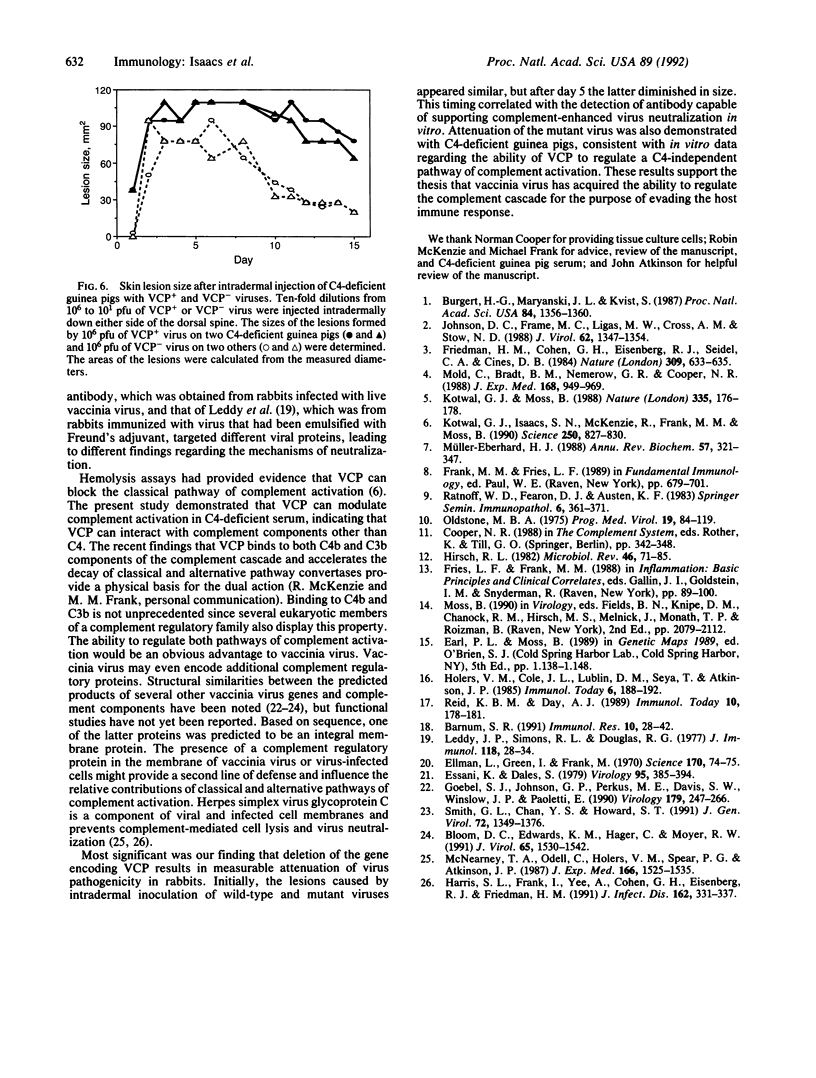
The role of a viral gene product in evasion of the host immune response was investigated. The antibody-dependent complement-enhanced neutralization of vaccinia virus infectivity was prevented by the culture medium from vaccinia virus-infected cells. The vaccinia virus complement-control protein (VCP) was identified as the secreted product of vaccinia virus gene C21L and has homology to a group of eukaryotic genes encoding regulators of complement activation. Thus, the culture medium from cells infected with a C21L deletion mutant was VCP deficient and had little or no effect on antibody-dependent complement-enhanced neutralization. In addition, the anticomplement effect was associated with the C21L-encoded protein partially purified from the medium of cells infected with wild-type virus. Antibody-dependent, complement-enhanced neutralization of vaccinia virus occurred with a complement source that was deficient in the classical pathway complement component C4 and required the alternative pathway complement factor B. Furthermore, the presence of VCP abrogated the complement-enhanced neutralization in C4-deficient serum. Together with previous hemolysis data, the present result suggests that VCP can inhibit both the classical and alternative pathways of complement activation. Skin lesions caused by the C21L deletion mutant were smaller than those caused by wild-type virus, demonstrating an important role for VCP in virulence. The C21L deletion mutant also was attenuated in C4-deficient guinea pigs, consistent with in vitro studies. Vaccinia virus appears to have acquired the ability to regulate the complement cascade for the purpose of evading the host immune response.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnum S. R. C4b-binding protein, a regulatory protein of complement. Immunol Res. 1991;10(1):28–42. doi: 10.1007/BF02918165. [DOI] [PubMed] [Google Scholar]
- Bloom D. C., Edwards K. M., Hager C., Moyer R. W. Identification and characterization of two nonessential regions of the rabbitpox virus genome involved in virulence. J Virol. 1991 Mar;65(3):1530–1542. doi: 10.1128/jvi.65.3.1530-1542.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman L., Green I., Frank M. Genetically controlled total deficiency of the fourth component of complement in the guinea pig. Science. 1970 Oct 2;170(3953):74–75. doi: 10.1126/science.170.3953.74. [DOI] [PubMed] [Google Scholar]
- Essani K., Dales S. Biogenesis of vaccinia: evidence for more than 100 polypeptides in the virion. Virology. 1979 Jun;95(2):385–394. doi: 10.1016/0042-6822(79)90493-8. [DOI] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Harris S. L., Frank I., Yee A., Cohen G. H., Eisenberg R. J., Friedman H. M. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990 Aug;162(2):331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L. The complement system: its importance in the host response to viral infection. Microbiol Rev. 1982 Mar;46(1):71–85. doi: 10.1128/mr.46.1.71-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Isaacs S. N., McKenzie R., Frank M. M., Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990 Nov 9;250(4982):827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988 Sep 8;335(6186):176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- Leddy J. P., Simons R. L., Douglas R. G. Effect of selective complement deficiency on the rate of neutralization of enveloped viruses by human sera. J Immunol. 1977 Jan;118(1):28–34. [PubMed] [Google Scholar]
- McNearney T. A., Odell C., Holers V. M., Spear P. G., Atkinson J. P. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987 Nov 1;166(5):1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold C., Bradt B. M., Nemerow G. R., Cooper N. R. Epstein-Barr virus regulates activation and processing of the third component of complement. J Exp Med. 1988 Sep 1;168(3):949–969. doi: 10.1084/jem.168.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Virus neutralization and virus-induced immune complex disease. Virus-antibody union resulting in immunoprotection or immunologic injury--two sides of the same coin. Prog Med Virol. 1975;19:84–119. [PubMed] [Google Scholar]
- Ratnoff W. D., Fearon D. T., Austen K. F. The role of antibody in the activation of the alternative complement pathway. Springer Semin Immunopathol. 1983;6(4):361–371. doi: 10.1007/BF02116280. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S., Howard S. T. Nucleotide sequence of 42 kbp of vaccinia virus strain WR from near the right inverted terminal repeat. J Gen Virol. 1991 Jun;72(Pt 6):1349–1376. doi: 10.1099/0022-1317-72-6-1349. [DOI] [PubMed] [Google Scholar]