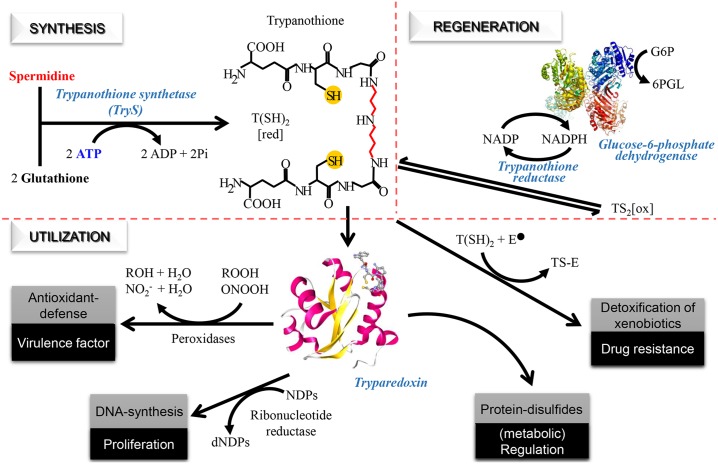
Fig 1. Trypanothione dependent redox metabolism.
The chemical structure of trypanothione (N1,N8-bis(glutathionyl)spermidine; T(SH)2) is depicted at the center. Synthesis: trypanothione synthetase catalyzes the ligation of two molecules of gluthatione to one of spermidine using the energy provided by two ATP molecules. Regeneration: trypanothione reductase maintains trypanothione in the reduced state at expenses of NADPH, which can be supplied by the oxidative phase of the pentose phosphate pathway via glucose 6-phosphate dehydrogenase. Utilization: reduced trypanothione is involved in multiple functions such as the detoxification of xenobiotics, cell proliferation, defense against oxidants and protein thiol-redox homeostasis. The multipurpose oxidoreductase tryparedoxin plays an important role catalyzing electron transfer from T(SH)2 to different molecular targets (e.g. peroxidases, ribonucleotide reductase and protein disulfides). G6P: glucose-6-phosphate, 6PGL: 6-phosphogluconolactone, T(SH)2: reduced trypanothione, TS2[ox]: oxidized trypanothione, NDPs: nucleosides diphosphate, dNDP: deoxinucleosides diphosphate, E-: electrophilic species, TS-E: trypanothione-electrophile adduct, ROOH: hydroperoxide, ONOOH: peroxynitrite, NO2-: nitrite.