Figure 6.
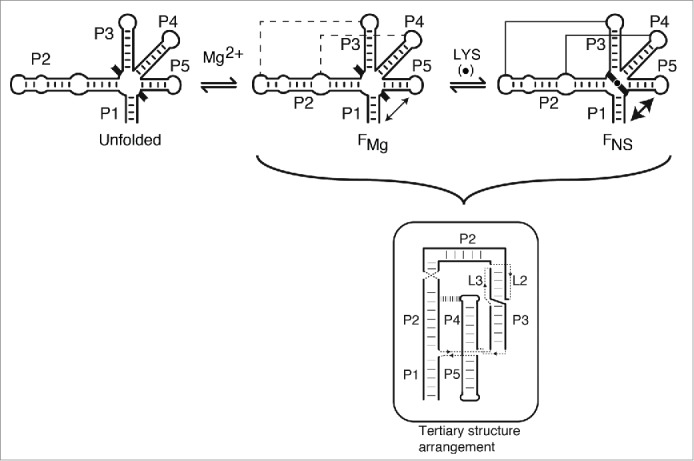
Proposed folding pathway and ligand sensing mechanism of the lysC riboswitch aptamer. In absence of Mg2+ ions, the lysC aptamer is assumed to adopt an unfolded structure (unfolded) in which only secondary structure elements (P1 to P5 stems) are formed. The presence of black braces pointing outside the aptamer domain indicates that junction core residues are not folded at this stage. Upon Mg2+ ions binding, the aptamer is reorganized into a structure (FMg) exhibiting long-range tertiary interactions L2-L3 and P2-L4 (dotted lines), as observed in crystal structures. Mg2+ ions binding also promote the formation of the P1-P5 folding transition (double arrow). Addition of lysine (LYS, black circle) results in the adoption of the native state (FNS) that consists of an additional P1-P5 transition as well as the reorganization of the junction core residues (inward orientation of black braces).
