Table 2.
Reaction with Morpholinea
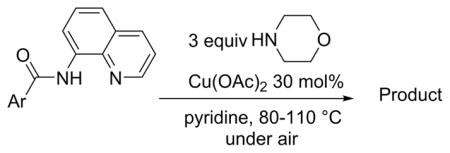
| |||
|---|---|---|---|
| entry | Ar | product | yield, % |
| 1 | Ph |

|
95 |
| 2 | 2-naphthyl |
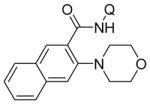
|
62 |
| 3 | 4-ClC6H4 |
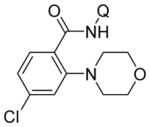
|
71 |
| 4 | 4-NCC6H4 |

|
78 |
| 5 | 4-CF3OC6H4 |
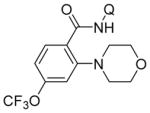
|
87 |
| 6 | 4-CF3SC6H4 |

|
86 |
| 7 | 3-NO2C6H4 |

|
73 |
| 8 | 3-CH3OC6H4 |
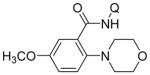
|
72 |
| 9 | 4-vinylC6H4 |
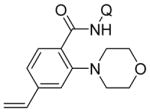
|
78 |
| 10 | 4-SO2NPr2-C6H4 |
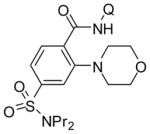
|
76 74b |
| 11 | 4-pyridyl |
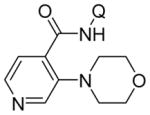
|
75 |
| 12 | 3-pyridyl |
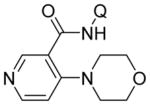
|
77 |
| 13 | 4-(3,5-di-methyl-1H-pyrazol-1-yl)C6H4 |

|
90 |
| 14 | 2-thio-phenyl |
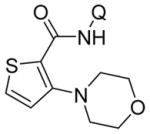
|
65 |
| 15c | 3-F-C6H4 |

|
39 |

|
36 | ||
Amide (1.0 mmol), Cu(OAc)2 (0.3 mmol), pyridine (2.0 mL), morpholine (3.0 mmol), 5–36 h, 80–110 °C. Yields are isolated yields. Please see Supporting Information for details.
Reaction scale of 5 mmol.
Approximately 1:1 ratio of isomers formed.
