Table 3.
Amination with Secondary Aminesa

| |||
|---|---|---|---|
| entry | amine | product | yield, % |
| 1 | N-Bn-piperazine |
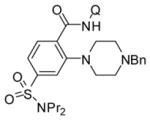
|
63 |
| 2 | N-2-furoyl-piperazine |
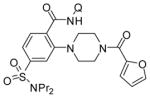
|
64 |
| 3 | 4-morpholinyl-piperidine |

|
73 |
| 4 | 4-(N-Boc)-amino-piperidine |

|
67 |
| 5 | nornicotine |

|
47 |
| 6 | methylbenzylamine |
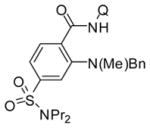
|
73 |
| 7 | 4-MeO-methylbenzylamine |

|
77 |
Amide (1.0 mmol), Cu(OAc)2 (0.3 mmol), pyridine (2.0 mL), amine (3.0 mmol), 5–36 h, 110 °C. Yields are isolated yields. Please see Supporting Information for details. PMB = p-methoxybenzyl.
